How to use Cervidil
Leaflet accompanying the packaging: patient information
Cervidil, 10 mg, intravaginal therapeutic system
Dinoprostone
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or nurse. See section 4.
- Cervidil should only be used under the supervision of an appropriate specialist.
Table of contents of the leaflet:
- 1. What Cervidil is and what it is used for
- 2. Important information before using Cervidil
- 3. How to use Cervidil
- 4. Possible side effects
- 5. How to store Cervidil
- 6. Contents of the packaging and other information
1. What Cervidil is and what it is used for
Cervidil contains the active substance dinoprostone 10 mg and is used to facilitate the initiation of the labor process, provided that 37 weeks of pregnancy have been completed. Dinoprostone dilates the part of the birth canal called the cervix, allowing the baby to pass through. There may be several reasons why the patient may need help starting this process. You should ask your doctor if you would like more information.
2. Important information before using Cervidil
When not to use Cervidil
Cervidil will not be administered:
- if the size of the baby's head may cause problems during delivery
- if the baby is not in the correct position in the uterus to allow for a natural birth
- if the baby's health is unsatisfactory and/or if the baby is at risk
- if the patient has had a previous major surgery or cervical tear
- if the patient has an untreated inflammatory disease in the pelvic area (infection of the uterus, ovaries, fallopian tubes, and/or cervix)
- if the placenta covers the birth canal
- if the patient has unexplained vaginal bleeding during the current pregnancy
- if the patient has had previous uterine surgery, including cesarean sections
- if the patient is allergic to dinoprostone or any of the other ingredients of this medicine (listed in section 6).
The doctor or nurse will not administer Cervidil or will remove the medicine if it has already been administered:
- after the onset of labor
- if the patient requires administration of a medicine, such as a medicine containing oxytocin, to support the progress of labor
- if the contractions are too strong or prolonged
- if the baby is at risk
- if the patient experiences side effects (see section 4. Possible side effects).
Experience with the use of Cervidil in patients with ruptured membranes is limited. If Cervidil has already been administered, the doctor or nurse will remove the medicine when the membranes rupture or are planned to be ruptured by the doctor or nurse.
Warnings and precautions
Before starting treatment with Cervidil, tell your doctor or nurse if any of the following apply to you:
- if you have or have had asthma (breathing difficulties) or glaucoma (eye disease)
- if you have had strong or prolonged contractions in a previous pregnancy
- if you have a lung, liver, or kidney disease
- if you have more than one child
- if you have had more than three full-term births
- if you are taking a pain reliever and/or anti-inflammatory medicine containing non-steroidal anti-inflammatory drugs (also known as NSAIDs), such as aspirin
- if you are 35 or older, if you have had complications during pregnancy, such as diabetes, high blood pressure, and low thyroid hormone levels (hypothyroidism), or if your pregnancy has lasted longer than 40 weeks due to an increased risk of developing disseminated intravascular coagulation - a rare disease that affects blood clotting.
Children and adolescents
Cervidil has not been studied in children and adolescents under the age of 18.
Cervidil and other medicines
Tell your doctor or nurse about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take. Cervidil may make you more sensitive to medicines that stimulate uterine contractions, which are used to strengthen contractions. It is not recommended to administer these medicines together with Cervidil.
Pregnancy and breastfeeding
Cervidil is used to facilitate the initiation of labor at term. Cervidil should not be used during other periods of pregnancy. The use of Cervidil during breastfeeding has not been studied. Cervidil may pass into breast milk, but it is assumed that the amounts and duration of passage are limited and should not pose a barrier to breastfeeding. No effect on breastfed newborns has been observed.
Driving and using machines
This does not apply, as Cervidil is only intended for use in connection with labor.
3. How to use Cervidil
Cervidil will be administered to you by trained medical staff in a hospital or clinic equipped with devices to monitor your condition and that of your baby. The doctor or nurse will insert one intravaginal therapeutic system (vaginal insert) into your vagina, near the cervix. You will not do this yourself. Before insertion into the vagina, the doctor or nurse may coat the vaginal insert with a small amount of lubricating gel. A tape of suitable length will remain outside the vagina to facilitate removal of the vaginal insert when necessary. You will be lying down during this procedure and will need to remain in a lying position for about 20-30 minutes after the insertion of the Cervidil vaginal insert. After insertion into the vagina, the vaginal insert absorbs moisture, allowing for the slow release of dinoprostone. While the vaginal insert is in the vagina, helping to initiate labor, you will be regularly monitored, including:
- cervical dilation,
- uterine contractions,
- labor pain and the baby's condition.
Depending on the progress of labor, the doctor or nurse will decide how long Cervidil should remain in the vagina. Cervidil may remain in the vagina for up to 24 hours. When removing the vaginal insert from the vagina, it will be 2-3 times larger than its initial size and will be elastic.
Using Cervidil for a longer period than recommended
If Cervidil is used for a longer period than recommended, it may lead to intensified uterine contractions or put the baby at risk. The Cervidil vaginal insert will then be removed immediately.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Common:may affect up to 1 in 10 people:
- Intensified uterine contractions with or without an effect on the baby's condition
- The baby's health may be at risk and/or the baby's heart rate may be faster or slower than normal
- Change in the color of the amniotic fluid
Uncommon:may affect up to 1 in 100 people:
- Headache
- Decreased blood pressure
- Newborn has difficulty breathing immediately after birth
- Newborn has high bilirubin levels, a product of red blood cell breakdown, which can cause yellowing of the skin and eyes
- Itching
- Excessive vaginal bleeding after delivery
- Placenta separates from the uterine wall before the baby is born
- Newborn has low overall condition immediately after birth
- Slow progress of labor
- Inflammation of the membranes lining the uterus
- Uterus does not contract after delivery due to lack of normal uterine contractions
- Burning sensation in the genital area
- Fever
Frequency not known: frequency cannot be estimated from the available data
- Fetal death, stillbirth, and newborn death; especially after severe events, such as uterine rupture.
- Disseminated intravascular coagulation - a rare disease that affects blood clotting. This can cause blood clots to form and may increase the risk of bleeding.
- Amniotic fluid, which surrounds the baby during pregnancy, may enter the mother's bloodstream during labor and block blood vessels, leading to a condition called amniotic fluid embolism, which can include symptoms such as: shortness of breath, low blood pressure, anxiety, and chills; life-threatening coagulation disorders, seizures, coma, bleeding, and fluid in the lungs, life-threatening risk to the fetus, such as slow heart rate.
- Hypersensitivity reactions and severe allergic reactions (anaphylactic reaction), which can include: difficulty breathing, shortness of breath, weak and rapid pulse, dizziness, itching, redness of the skin, and rash.
- Abdominal pain
- Nausea
- Vomiting
- Diarrhea
- Itching in the genital area
- Uterine rupture
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Aleje Jerozolimskie 181C, 02-222 Warsaw, Tel: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Cervidil
Keep the medicine out of the sight and reach of children. Do not use this medicine after the expiry date stated on the foil sachet and carton after the words EXP. The expiry date refers to the last day of the month stated. The batch number is stated after the word (Lot). Store in a freezer (between -10°C and -25°C). Store in the original packaging to protect from moisture. Medicines should not be disposed of via wastewater or household waste. After use, the doctor or nurse will dispose of the entire product as clinical waste. This will help protect the environment.
6. Contents of the packaging and other information
What Cervidil contains
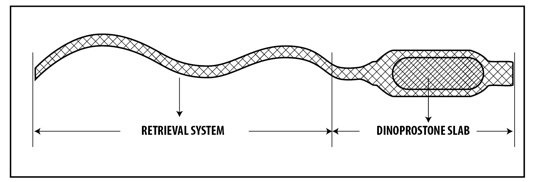
- The active substance of Cervidil is dinoprostone, also known as Prostaglandin E2. Each intravaginal therapeutic system contains 10 mg of dinoprostone, which is released at a rate of approximately 0.3 mg per hour over 24 hours. The other ingredients are:
- Hydrogel polymer containing: macrogol 8000, 1,2,6-hexanetriol, 4,4’-diisocyanatocyclohexane, iron chloride
- Polyester thread
What Cervidil looks like and contents of the packaging
The intravaginal therapeutic system is a small, rectangular plastic insert, placed in an applicator made of fabric used for removing the insert from the vagina. The plastic insert is a hydrogel polymer that swells in the presence of moisture and releases dinoprostone. The applicator has a long tape that allows the doctor or nurse to remove the insert from the vagina when necessary. Each intravaginal therapeutic system is individually placed in a tightly closed foil sachet made of laminated aluminum/polyethylene tape and is packaged in a carton. The packaging contains 5 intravaginal therapeutic systems.
Marketing authorization holder and manufacturer
Marketing authorization holder
Ferring GmbH, Wittland 11, D-24109 Kiel, Germany
Manufacturers
Ferring GmbH, Wittland 11, D-24109 Kiel, Germany
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Austria: PROPESS 10 mg vaginales Freisetzungssystem, Belgium: PROPESS 10 mg hulpmiddel voor vaginaal gebruik, PROPESS 10 mg système de diffusion, PROPESS 10 mg vaginales Wirkstofffreisetzungssystem, Bulgaria: ПРОПЕС 10 mg вагинална лекарстводоставяща система / PROPESS 10 mg vaginal delivery system, Croatia: Propess 10 mg sustav za isporuku u rodnicu, Cyprus: Propess, Czech Republic: CERVIDIL, Denmark: Propess, vaginalindlæg, Estonia: Propess, Finland: Propess 10 mg depotlääkevalmiste, emättimeen, France: PROPESS 10 mg, système de diffusion vaginal, Germany: PROPESS 10 mg vaginales Freisetzungssystem, Greece: PROPESS 10 mg σύστημα ενδοκολπικής χορήγησης, Hungary: Propess 10mg hüvelyben alkalmazott gyógyszerleadó rendszer, Ireland: Propess 10 mg vaginal delivery system, Italy: PROPESS 10 mg – Dispositivo vaginale, Latvia: Propess, Lithuania: Propess, Luxembourg: PROPESS 10 mg système de diffusion vaginal, Netherlands: Propess, vaginaal toedieningssysteem 10 mg, Norway: Propess, Poland: Cervidil, Portugal: PROPESS 10 mg, Sistema de libertação vaginal, Romania: PROPESS 10 mg/24 ore sistem cu cedare vaginală, Slovakia: Cervidil 10 mg vaginálny inzert, Slovenia: Propess 10 mg vaginalni dostavni sistem, Spain: PROPESS 10 mg sistema de liberación vaginal, Sweden: Propess 10 mg vaginalinlägg, United Kingdom (Northern Ireland): PROPESS 10 mg vaginal delivery system
Date of last revision of the leaflet:09/2021 --------------------------------------------------------------------------------------------------------------------------------Information intended for healthcare professionals only:
INSTRUCTIONS FOR USE
Insertion
- 1. To remove Cervidil from the packaging, first break the foil along the top edge of the sachet. Do not use scissors or sharp objects to cut the foil, as this may damage the product. Hold the applicator and gently pull the product out of the sachet. Holding the intravaginal therapeutic system between your index and middle fingers, insert it into the vagina. If necessary, a small amount of water-soluble lubricant can be used.
- 2. Cervidil is inserted transversely, high in the posterior fornix of the vagina.
- 3. Leave a portion of the tape (about 2 cm) protruding from the vagina to facilitate easy removal of the intravaginal therapeutic system. If necessary, the tape can be shortened further.
- 4. Ensure that the patient lies down or sits for 20-30 minutes after insertion to allow the insert to swell.
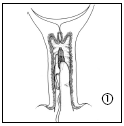
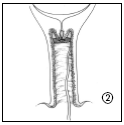
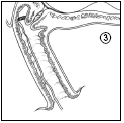
Removal
Cervidil can be quickly and easily removed by gently pulling the tape of the applicator. After removal, ensure that the entire product (intravaginal therapeutic system and applicator) has been removed from the vagina.
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterFerring GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CervidilDosage form: Gel, 0.5 mg/3 gActive substance: dinoprostonePrescription not requiredDosage form: Gel, 500 mcg/3 gActive substance: dinoprostonePrescription not requiredDosage form: Tablets, 25 mcgActive substance: misoprostolPrescription required
Alternatives to Cervidil in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Cervidil in Ukraine
Alternative to Cervidil in Spain
Online doctors for Cervidil
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Cervidil – subject to medical assessment and local rules.