PROPESS 10 mg VAGINAL DELIVERY SYSTEM

How to use PROPESS 10 mg VAGINAL DELIVERY SYSTEM
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
P
Dinoprostone
Read all of this leaflet carefully before you start using this medicine because it contains important information for you
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
- Propess must be administered by healthcare professionals, under the supervision of a specialist.
Contents of the Package Leaflet
- What is Propess and what is it used for
- What you need to know before you start using Propess
- How to use Propess
- Possible side effects
- Storage of Propess
- Contents of the pack and other information
1. What is Propess and what is it used for
Propess contains the active substance dinoprostone 10 mg and is used to help start the process of labor, provided that 37 weeks of gestation have been completed. Dinoprostone dilates the cervix to allow the baby to be born. There are several reasons why help may be needed to start this process. Ask your doctor if you want to know more.
2. What you need to know before you start using Propess
Do not use Propess
Propess must not be used:
- if the size of your baby's head is too large and may cause problems during delivery
- if your baby is not in the correct position inside the uterus for a normal delivery
- if your baby is unwell and/or suffering
- if you have had a major surgery or rupture of the cervix (womb) in the past
- if you have untreated inflammatory diseases of the pelvis (infection of the uterus, ovaries, fallopian tubes and/or cervix)
- if the placenta blocks the birth canal
- if you have had unexplained vaginal bleeding during this pregnancy
- if you have had a previous cesarean section or other uterine surgery
- if you are allergic (hypersensitive) to dinoprostone or any of the other components of Propess (listed in section 6).
Your doctor or midwife should not use Propess or, if they have already done so, should remove it:
- when labor has started
- if you are given a medicine, for example an oxytocic (uterine stimulant), to help with the progress of labor
- if your contractions are too strong or prolonged
- if your baby starts to suffer
- if side effects occur (see section 4).
Experience with the use of Propess in women who have broken waters is limited. Your doctor or midwife will remove it if your waters break naturally or artificially.
Warnings and Precautions
Before using Propess, please inform your doctor or midwife if you have any of the following:
- if you have or have had asthma (difficulty breathing) or glaucoma (an eye disease)
- if you have had very strong or prolonged contractions in a previous pregnancy
- if you have any lung, liver, or kidney disease
- if you have a multiple pregnancy
- if you have given birth to more than three children
- if you are taking pain and/or anti-inflammatory medicines that contain non-steroidal anti-inflammatory drugs (NSAIDs), for example aspirin
- if you are 35 years or older, if you have had complications during pregnancy such as diabetes, high blood pressure, and low thyroid hormone levels (hypothyroidism), or if your pregnancy goes beyond 40 weeks, due to a higher risk of developing disseminated intravascular coagulation (DIC), a rare disease that affects blood clotting.
Children and Adolescents
The use of Propess has not been investigated in girls and adolescents under 18 years of age.
Using Propess with other medicines:
Tell your doctor or pharmacist if you are using or have recently used any other medicines, including those obtained without a prescription. Propess may make you more sensitive to other medicines in the oxytocic group that are used to strengthen contractions. It is not recommended to administer these medicines together with Propess.
Pregnancy and Breastfeeding
Propess is used from the 38th week of gestation to help start the process of labor. Propess should not be used in other stages of pregnancy.
Propess has not been investigated during breastfeeding. Propess may be excreted in breast milk, but it is expected that the level and duration will be very limited and will not interfere with breastfeeding. No effects have been observed in breastfed babies.
Driving and using machines
Not relevant since Propess should only be used in relation to labor.
3. How to use Propess
Propess will be administered by a trained professional in a hospital or clinic where facilities are available to monitor you and your baby.
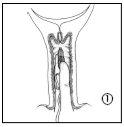
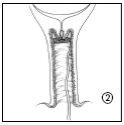
The doctor or midwife will insert the vaginal delivery system into your vagina, near the cervix. You should not do this yourself. Before applying Propess, the doctor or midwife will cover the vaginal delivery system with a small amount of gelatinous lubricant. Enough tape should be left outside the vagina to facilitate the removal of the vaginal delivery system when necessary.
You should remain lying down during the insertion of Propess and should remain in this position for the following 20-30 minutes.
After insertion, the vaginal delivery system absorbs some of the moisture from the area, allowing the dinoprostone to be released slowly.
Once the vaginal delivery system is in place to help start labor, you will be periodically monitored, among other things, for the following parameters:
- Cervical maturation
- Uterine contractions
- Labor pains and monitoring of the baby's health status
The doctor or midwife will decide how long you need to have Propess in place based on your progress. Propess can remain in place for a maximum of 24 hours.
When removing the product from the vagina, the vaginal delivery system will have expanded to a size 2-3 times larger than the original, and will be flexible.
If you use more Propess than you should
If you have used Propess for longer than you should, there may be an increase in contractions or fetal distress. The Propess vaginal delivery system should be removed immediately.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common: may affect up to 1 in 10 people
- increase in uterine contractions that may or may not affect the baby.
- the baby may experience distress and/or an abnormal heart rate.
- Discoloration of the amniotic fluid.
Uncommon: may affect up to 1 in 100 people
- Headache
- Decrease in blood pressure
- The baby has difficulty breathing immediately after birth
- The baby has high levels of bilirubin in the blood, a waste product of red blood cells, which can cause yellowing of the skin and eyes.
- Itching
- Severe vaginal bleeding after delivery
- The placenta separates from the uterine wall before the baby is born
- Decrease in the general condition of the newborn immediately after birth
- Slow progress of labor
- Inflammation of the membranes that line the inside of the uterus
- The mother's uterus does not contract after delivery due to a lack of normal uterine contractions
- Burning sensation in the genital area
- Fever
Frequency not known: frequency cannot be estimated from the available data:
- Fetal death, stillbirth, and neonatal death; especially after severe events such as uterine rupture.
- Disseminated intravascular coagulation (DIC); a rare disease that affects blood clotting, causing clots and increasing the risk of bleeding after delivery.
- The fluid that surrounds the baby during pregnancy can enter the mother's bloodstream during delivery and block a blood vessel, leading to a condition called amniotic fluid embolism, which can include symptoms such as: shortness of breath, low blood pressure, anxiety, and chills, life-threatening problems with blood clotting, convulsions, coma, bleeding, and fluid in the lungs, fetal distress of the type low heart rate.
- Severe allergic reactions, including difficulty breathing, shortness of breath, weak or rapid pulse, dizziness, itching, redness of the skin, and severe rash.
- Abdominal pain
- Nausea
- Vomiting
- Diarrhea
- Swelling of the genital area
- Uterine rupture
Reporting of side effects:
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Monitoring System for Human Use: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Propess
Keep out of the sight and reach of children.
Do not use Propess after the expiry date which is stated on the packaging and on the aluminum pouch. The expiry date is the last day of the month stated.
Store in a freezer (-10 to -25°C). Store in the original package to protect from moisture.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Further information
Composition of Propess:
- The active substance is dinoprostone, commonly known as prostaglandin E2. Each vaginal delivery system contains 10 milligrams of dinoprostone, which is released at approximately 0.3 milligrams per hour for 24 hours.
- The other components are: cross-linked macrogol polymer (hydrogel) and polyester thread.
Appearance and packaging of the product
The vaginal delivery system is a small rectangular piece of plastic in a reticulated extraction system.
The plastic piece is a hydrogel polymer that swells in the presence of moisture, releasing dinoprostone.
The extraction system has a long tape that allows the doctor or midwife to remove it when necessary.
Each vaginal delivery system is presented in an individual aluminum pouch made from a laminated aluminum strip and packaged in a pack.
Pack containing 5 vaginal delivery systems.
Marketing authorization holder and manufacturer
Marketing authorization holder:
FERRING S.A.U.
C/ del Arquitecto Sánchez Arcas nº 3, 1º
28040 Madrid, SPAIN.
Manufacturer:
Ferring GmbH
Wittland 11
24109 Kiel
Germany
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Austria, PROPESS 10 mg vaginal delivery system
Belgium, PROPESS 10 mg aid for vaginal use, PROPESS 10 mg vaginal delivery system, PROPESS 10 mg vaginal active substance release system
Bulgaria, ?????? 10 mg ????????? ?????????????????? ??????? / PROPESS 10 mg vaginal delivery system
Croatia, Propess 10 mg system for vaginal delivery
Cyprus, Propess
Czech Republic, CERVIDIL
Denmark, Propess, vaginal insert
Estonia, Propess
Finland, Propess 10 mg depot preparation for the vagina
France, PROPESS 10 mg, vaginal delivery system
Germany, PROPESS 10 mg vaginal delivery system
Greece, PROPESS 10 mg ?????????? ?????????? ?????????
Hungary, Propess 10mg vaginal drug delivery system
Ireland, Propess 10 mg vaginal delivery system
Italy, PROPESS 10 mg - Vaginal device
Latvia, Propess
Lithuania, Propess
Luxembourg, PROPESS 10 mg vaginal delivery system
Netherlands, Propess, vaginal delivery system 10 mg
Norway, Propess
Poland, Cervidil
Portugal, PROPESS 10 mg, Vaginal release system
Romania, PROPESS 10 mg/24 hours vaginal release system
Slovakia, Cervidil 10 mg vaginal insert
Slovenia, Propess 10mg vaginal delivery system
Spain, PROPESS 10 mg vaginal delivery system
Sweden, Propess 10 mg vaginal insert
United Kingdom (Northern Ireland), PROPESS 10mg vaginal delivery system
Date of last revision of this leaflet:September 2021.
----------------------------------------------------------------------------------------------------------------------
The following information is intended for healthcare professionals only:
INSTRUCTIONS FOR USE
Application
|
|
| |
|
|
|
Removal
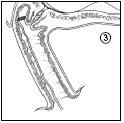
Propess can be quickly and easily removed by carefully pulling the tape. After removal, check that the entire product (vaginal delivery system and extraction system) has been removed from the vagina.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PROPESS 10 mg VAGINAL DELIVERY SYSTEMDosage form: INJECTABLE PERFUSION, 10mg/ml dinoprostoneActive substance: dinoprostoneManufacturer: Pfizer S.L.Prescription requiredDosage form: TABLET, 25 microgramsActive substance: misoprostolManufacturer: Norgine B.V.Prescription requiredDosage form: VAGINAL SUPPOSITORY/CAPSULE/TABLET, 200 µgActive substance: misoprostolManufacturer: Laboratorios Bial S.A.Prescription required
Online doctors for PROPESS 10 mg VAGINAL DELIVERY SYSTEM
Discuss questions about PROPESS 10 mg VAGINAL DELIVERY SYSTEM, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions