

Octanate

Ask a doctor about a prescription for Octanate

How to use Octanate
Leaflet accompanying the packaging: information for the user
Octanate, 50 IU/ml, powder and solvent for solution for injection
Human coagulation factor VIII Octanate, 100 IU/ml, powder and solvent for solution for injection
Human coagulation factor VIII
You should read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- You should consult a doctor, pharmacist, or nurse if you have any further questions.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm them, even if their symptoms are the same as yours.
- If any of the side effects get worse, or if you notice any not listed in the leaflet, you should tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet:
- 1. What is Octanate and what is it used for
- 2. Important information before using Octanate
- 3. How to use Octanate
- 4. Possible side effects
- 5. How to store Octanate
- 6. Contents of the packaging and other information
1. What is Octanate and what is it used for
Octanate belongs to a group of medicines called coagulation factors and contains human coagulation factor VIII. It is a special protein that increases the blood's ability to clot. Octanate is used to treat and prevent bleeding in patients with hemophilia A. This is a condition where bleeding is prolonged compared to what is expected. It results from a congenital deficiency of factor VIII in the blood.
2. Important information before using Octanate
It is strongly recommended that each time a dose of Octanate is administered to the patient, the name and batch number of the product should be recorded in order to retain information about the batch used. Your doctor may recommend considering vaccination against viral hepatitis A and B in case of regular or repeated administration of human factor VIII products.
When not to use Octanate
If the patient is allergic to human coagulation factor VIII or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting to take Octanate, you should consult a doctor, pharmacist, or nurse. Octanate contains trace amounts of other human proteins. Any medicine that contains proteins and is injected into a vein (given intravenously) may cause allergic reactions (see section 4. Possible side effects). The formation of inhibitors (antibodies) is a known complication that may occur during treatment with all factor VIII medicines. These inhibitors, especially at high concentrations, disrupt proper treatment, and the patient will be closely monitored for the production of these inhibitors. If the patient's bleeding is not properly controlled with Octanate, you should immediately tell your doctor.
Information about blood and plasma used to manufacture Octanate
During the manufacture of medicines from human blood or plasma, certain measures are taken to prevent the transmission of infections to patients. These include careful selection of blood and plasma donors to ensure the exclusion of donors who may be carriers of infections, as well as testing all collected samples and plasma pools for the presence of viruses/infections. The manufacturers of these products have implemented steps in the processing of blood or plasma that are designed to inactivate or remove viruses. Despite these measures, when administering medicines prepared from human blood or plasma, it cannot be completely excluded that the possibility of transmitting an infection. This also applies to unknown or newly emerging viruses, or other types of infections. The measures taken are considered effective against enveloped viruses, such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV), as well as non-enveloped hepatitis A virus (HAV). The methods used may have limited effectiveness against non-enveloped viruses such as parvovirus B19. Infection with parvovirus B19 can be dangerous for pregnant women (fetal infection) and for people whose immune system is suppressed or who suffer from certain types of anemia (e.g., sickle cell anemia or abnormal red blood cell breakdown).
Other medicines and Octanate
You should tell your doctor or pharmacist about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take, including those that are available without a prescription. No interactions between human factor VIII and other medicinal products are known. However, you should not mix Octanate with other medicines during infusion.
Pregnancy, breastfeeding, and fertility
If you are pregnant, breastfeeding, or think you may be pregnant, or plan to become pregnant, you should consult a doctor or pharmacist before using this medicine.
Driving and using machines
No effect on the ability to drive and use machines has been observed.
Octanate contains
For one vial of 250 IU: less than 1 mmol of sodium (23 mg) (main component of table salt) per vial, which may be considered "sodium-free". For one vial of 500 IU and 1000 IU: up to 40 mg of sodium (main component of table salt) per vial. This corresponds to 2% of the recommended daily maximum intake of 2 g of sodium for an adult.
3. How to use Octanate
Octanate should be administered intravenously after reconstitution in the attached solvent. Treatment should be started under medical supervision. Dosing in bleeding prophylaxis: In long-term prophylaxis of bleeding in patients with severe hemophilia A, factor VIII should be administered at a dose of 20 to 40 IU per kilogram of body weight at intervals of 2 to 3 days. The dose should be adjusted according to the clinical response. In some cases, it may be necessary to administer the medicine at shorter intervals or in higher doses.
Dose calculation:
Octanate should always be used according to the doctor's recommendations. In case of doubts, you should contact your doctor or pharmacist again. Factor VIII activity in plasma corresponds to the factor VIII content in plasma. It is expressed either as a percentage (compared to normal human plasma) or in international units (IU). The dose of factor VIII is expressed in IU. One International Unit (IU) of factor VIII activity is equivalent to the amount of factor VIII in 1 ml of normal human plasma. 1 IU of factor VIII per kilogram of body weight increases the activity of plasma factor VIII by 1.5% - 2% of normal activity. To calculate the required dose, you should determine the factor VIII activity in the patient's plasma. This will allow you to determine how much the activity should be increased. You should consult a doctor in case of uncertainty about how much the factor VIII activity in plasma should be increased and how to calculate the required dose. The required dose is calculated using the following formula: required number of units = body weight (kg) x required increase in factor VIII activity (%) (IU/dl) x 0.5 The amount to be administered and the frequency of administration of Octanate should always be adjusted according to the individual patient's clinical efficacy.
Administration in children
No special requirements for dosing in children have been found in clinical trials. Dosing is the same for adults and children, both for treatment and prophylaxis.
Home administration instructions
- You should read all the instructions and follow them carefully!
- Do not use the Octanate product after the expiration date stated on the label.
- During the procedure described below, you should maintain sterile conditions!
- Before administration, you should visually check if the prepared solution of the product does not contain solid particles or discoloration.
- The solution should be clear or slightly opalescent. Do not use solutions that are cloudy or contain solid particles.
- The prepared solution should be used immediately to prevent microbial contamination.
- Only use the supplied infusion set. Using other equipment for injections/infusions may cause additional risks and treatment failure.
Instructions for preparing the solution:
- 1. Do not use the product directly after removing it from the refrigerator. Leave the solvent and powder in closed vials until they reach room temperature.
- 2. Remove the caps from both vials and clean the rubber stoppers with one of the enclosed alcohol swabs.
- 3. The transfer set is shown in Fig. 1. Place the vial with the solvent on a flat surface and hold it firmly. Take the transfer set and turn it. Place the blue part of the transfer set on top of the vial with the solvent and press firmly until it clicks (Fig. 2 + 3). Do not turn during connection.
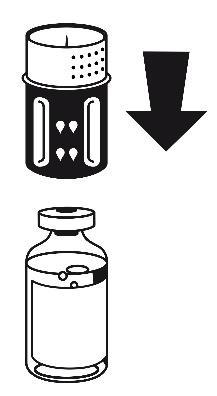
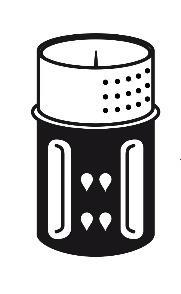
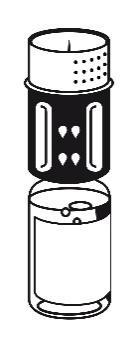
Fig. 1
Fig. 3
Fig. 2
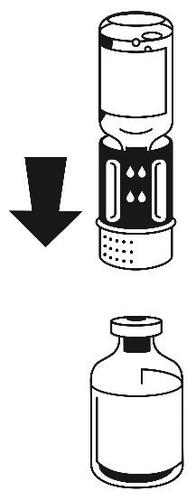
- 4.Place the vial with the powder on a flat surface and hold it firmly. Take the vial with the solvent with the attached transfer set and turn it upside down. Place the white part of the transfer set on top of the vial with the powder and press firmly until it clicks (Fig. 4). Do not turn during connection. The solvent flows automatically into the vial with the powder.
Fig. 4
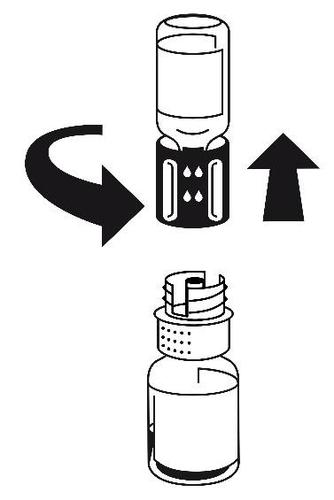
- 5.With both vials connected, gently rotate the vial with the powder until the product is dissolved. Dissolution is complete in less than 10 minutes at room temperature. During preparation, a slight foam may form. Unscrew the transfer set into two parts (Fig. 5). The foam will disappear.
Discard the empty vial with the solvent and the blue part of the transfer set.
Fig. 5
Instructions for performing the injection:
As a precaution, you should check your pulse before and during the injection. If you notice a significant increase in heart rate, you should reduce the injection rate or interrupt the administration of the medicine for a short time.
- 1. Connect the syringe to the white part of the transfer set. Turn the vial upside down and draw the solution into the syringe (Fig. 6). The solution should be clear or slightly opalescent. After transferring the solution, hold the syringe plunger firmly (with the needle facing down) and remove the syringe from the transfer set (Fig. 7).
Discard the empty vial with the transfer set.
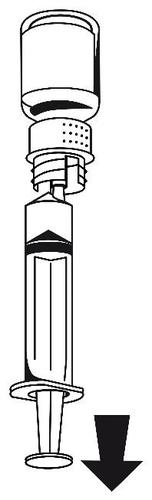
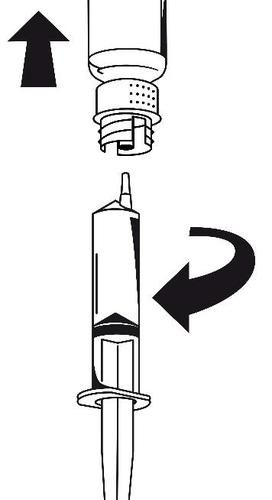
Fig. 6
Fig. 7
- 2. Clean the selected injection site with one of the enclosed alcohol swabs.
- 3. Connect the supplied infusion set to the syringe.
- 4. Insert the needle into the selected vein. If a tourniquet is used to make the vein visible, it should be loosened before starting the injection of Octanate.
- 5. Blood must not enter the syringe due to the risk of forming fibrin clots.
- 6. Inject the solution slowly into the vein, no faster than 2-3 ml per minute. If more than one vial of Octanate powder is used during a single administration, it is possible to reuse the same infusion set and syringe. The transfer set is intended for single use only.
Any unused product or waste material should be disposed of in accordance with local regulations.
Using more than the recommended dose of Octanate
No symptoms of overdose of human factor VIII have been observed. However, it is recommended not to exceed the recommended dose.
Missing a dose of Octanate
You should not use a double dose to make up for a missed dose. You should proceed to the next dose and continue dosing as recommended by your doctor or pharmacist. You should contact your doctor or pharmacist if you have any other questions about using this product.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Although they are rare (may affect up to 1 in 1,000 people), allergic or hypersensitivity reactions have been observed in patients treated with factor VIII products. You should contact your doctor if you experience any of the following symptoms: vomiting, burning sensation and painful sensation at the injection site, feeling of pressure in the chest, chills, tachycardia, nausea, tingling sensation, redness, headache, hives, low blood pressure, rash, anxiety, swelling of the face, lips, mouth, tongue, or throat, which may lead to difficulty swallowing or breathing (angioedema), fatigue (lethargy), wheezing. Very rarely(may affect up to 1 in 10,000 people), hypersensitivity may lead to a life-threatening allergic reaction called anaphylaxis, which may include shock, as well as any of the above symptoms. In such a case, you should immediately contact your doctor or call emergency services. Other rare side effects (may affect up to 1 in 1,000 people) include: Fever In children who have not been previously treated with factor VIII products, inhibitory antibodies (see section 2) may develop very frequently (more than 1 in 10 patients). However, in patients who have been previously treated with factor VIII (more than 150 days of treatment), the risk is not very common (less than 1 in 100 patients). If this happens, the patient's medicines may stop working properly, and the patient may experience persistent bleeding. If this happens, you should immediately contact your doctor. Information related to viral safety, see section 2. (You should be particularly careful when using Octanate). Reporting side effects If you experience any side effects, including any not listed in the leaflet, you should tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, PL–02 222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309, e-mail: [email protected] Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Octanate
The medicine should be stored out of sight and reach of children. Do not use this medicine after the expiration date stated on the label. The expiration date refers to the last day of the stated month. Store in a refrigerator (2°C – 8°C). Do not freeze. Store in the outer packaging to protect from light. The solution after reconstitution should be used immediately and only during a single administration. Do not use this medicine if you notice that the solution is cloudy or not completely dissolved. Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Octanate contains
The active substance of the medicine is human coagulation factor VIII.
Volume and concentrations
| Size of the Octanate powder vial (IU of factor VIII) | Size of the solvent vial (ml) (to be added to the Octanate powder vial) | Nominal concentration of the solution after reconstitution (IU of factor VIII/ml) |
| 250 IU | 5 | 50 |
| 500 IU | 10 | 50 |
| 1000 IU | 10 | 100 |
The other ingredients are: For the powder: sodium citrate, sodium chloride, calcium chloride, glycine For the solvent: water for injections
What Octanate looks like and what the pack contains
Octanate comes in the form of a powder and solvent for solution for injection. The powder is white or pale yellow and may also appear as a lump. The solvent is a clear, colorless liquid. 3 available pack sizes differ in factor VIII content and solvent: 250 IU/vial: after reconstitution in 5 ml, the strength is 50 IU/ml 500 IU/vial: after reconstitution in 10 ml, the strength is 50 IU/ml 1000 IU/vial: after reconstitution in 10 ml, the strength is 100 IU/ml All pack sizes contain: 1 package with equipment for intravenous injection (1 transfer set, 1 infusion set, 1 single-use syringe) 2 alcohol swabs.
Not all pack sizes may be available on the market.
Marketing authorization holder
Octapharma (IP) SPRL Allée de la Recherche 65 1070 Anderlecht Belgium For more detailed information, you should contact the representative of the marketing authorization holder.
Manufacturer
Octapharma Pharmazeutika Produktionsges.m.b.H Oberlaaer Str. 235 1100 Vienna Austria Octapharma S.A.S 70 – 72 Rue du Maréchal Foch BP 33 67381 Lingolsheim France Octapharma AB 112 75 Stockholm Sweden Date of last revision of the leaflet:26.03.2021
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterOctapharma AB Octapharma Pharmazeutika Produktionsgesellschaft mbH (OPG) Octapharma S.A.S.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OctanateDosage form: Powder, 1000 IUActive substance: coagulation factor VIIIManufacturer: CSL Behring GmbHPrescription requiredDosage form: Powder, 2000 IUActive substance: coagulation factor VIIIManufacturer: CSL Behring GmbHPrescription requiredDosage form: Powder, 250 IUActive substance: coagulation factor VIIIManufacturer: CSL Behring GmbHPrescription required
Alternatives to Octanate in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Octanate in Spain
Alternative to Octanate in Ukraine
Online doctors for Octanate
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Octanate – subject to medical assessment and local rules.














