
REFACTO AF 250 IU, POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use REFACTO AF 250 IU, POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
ReFacto AF 250 UI powder and solvent for solution for injection
ReFacto AF 500 UI powder and solvent for solution for injection
ReFacto AF 1000 UI powder and solvent for solution for injection
ReFacto AF 2000 UI powder and solvent for solution for injection
moroctocog alfa (recombinant human coagulation factor VIII)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information:
- What is ReFacto AF and what is it used for
- What you need to know before you use ReFacto AF
- How to use ReFacto AF
- Possible side effects
- Storing ReFacto AF
- Contents of the pack and further information
1. What is ReFacto AF and what is it used for
ReFacto AF contains the active substance moroctocog alfa, human coagulation factor VIII. Factor VIII is necessary for blood to clot and stop bleeding. In patients with hemophilia A (congenital factor VIII deficiency), it is either not present or does not work properly.
ReFacto AF is used to treat and prevent (prophylaxis) bleeding episodes in adults and children of all ages (including newborns) with hemophilia A.
2. What you need to know before you use ReFacto AF
Do not use ReFacto AF
- if you are allergic to moroctocog alfa or any of the other ingredients of this medicine (listed in section 6);
- if you are allergic to hamster proteins.
Consult your doctor if you are unsure.
Warnings and precautions
Consult your doctor or pharmacist before starting treatment with ReFacto AF
- if you have allergic reactions. Some signs of allergic reactions are difficulty breathing, shortness of breath, swelling, hives, itching, chest tightness, wheezing, and low blood pressure. Anaphylaxis is a severe allergic reaction that causes difficulty swallowing or breathing, redness or swelling of the hands, face, or both. If any of these symptoms occur, stop the infusion immediately and contact your doctor or seek immediate emergency medical attention. In cases of severe allergic reactions, alternative treatment should be considered.
- the formation of inhibitors (antibodies) is a known complication that can occur during treatment with all factor VIII medicines. These inhibitors, especially at high levels, can prevent the treatment from working properly, so you or your child will be closely monitored for the development of such inhibitors. If your bleeding or your child's bleeding is not being controlled with ReFacto AF, consult your doctor immediately.
- if your bleeding does not stop as expected, contact your doctor or seek immediate emergency medical attention.
Other medicines and ReFacto AF
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
ReFacto AF has no influence on the ability to drive and use machines.
ReFacto AF contains sodium
After reconstitution, ReFacto AF contains 1.27 mmol (or 29 mg) of sodium (the main component of cooking/table salt) per vial. This is equivalent to 1.5% of the maximum recommended daily intake of sodium for an adult. Depending on your body weight and your dose of ReFacto AF, you may receive multiple vials. This should be taken into account if you are on a low-salt diet.
3. How to use ReFacto AF
Follow the instructions for administration of this medicine exactly as prescribed by your doctor. If you are unsure, consult your doctor or pharmacist again.
Treatment with ReFacto AF should be started by a doctor experienced in the treatment of patients with hemophilia A. Your doctor will decide the dose of ReFacto AF you should receive. This dose and its duration will depend on your individual needs for factor VIII replacement therapy. ReFacto AF is administered by injection into a vein and the injection takes several minutes. ReFacto AF injections can be administered by patients or their caregivers, provided they have received appropriate training.
During your treatment, your doctor may change the dose of ReFacto AF you receive.
Consult your doctor before traveling. When traveling, you should carry your factor VIII product in sufficient quantity for the planned treatment.
It is recommended that each time ReFacto AF is administered, you record the name on the label and the batch number of the product. You can stick one of the detachable labels found on the vial to document the batch number in your diary or to report any side effects.
Reconstitution and administration
The instructions provided below are a guide for the reconstitution and administration of ReFacto AF. Patients should follow the specific reconstitution and administration instructions provided by their doctors.
For reconstitution, use only the pre-filled syringe provided in the package. For administration, other sterile disposable syringes can be used.
ReFacto AF is administered by intravenous (IV) infusion after reconstituting the lyophilized powder for injection with the provided syringe of solvent, [a 9 mg/ml (0.9%) sodium chloride injection solution]. ReFacto AF should not be mixed with other infusion solutions.
Always wash your hands before performing reconstitution and administration. During the reconstitution procedure, aseptic technique (i.e., clean and germ-free) should be followed.
Reconstitution:
- Wait for the ReFacto AF vial and the pre-filled syringe of solvent to reach room temperature.
- Remove the plastic cap from the ReFacto AF vial to expose the central part of the rubber stopper.
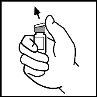
- Clean the top of the vial with the provided alcohol swab or another antiseptic solution and wait for it to dry. After cleaning, do not touch the rubber stopper with your hand or let it touch any surface.
- Remove the cap from the package of the plastic vial adapter. Do not remove the adapter from the package.
- Place the vial on a flat surface. While holding the package of the adapter, place the adapter over the vial and press firmly on the package until the adapter snaps into place on top of the vial and the adapter's spike penetrates the vial's stopper.
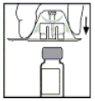
- Remove the package of the adapter and discard it.
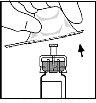
- Attach the plunger rod to the syringe of solvent by inserting the rod into the syringe's cap opening, pressing, and turning firmly until it is seated in the cap.
- Remove the plastic tip cap from the syringe of solvent by breaking the perforation of the cap. This is done by bending the cap up and down until the perforation breaks. Do not touch the inside of the cap or the tip of the syringe. The cap may need to be replaced (if ReFacto AF reconstituted is not administered immediately), so it should be set aside on its top.
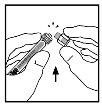
- Place the vial on a flat surface. Connect the syringe of solvent to the vial adapter by inserting the syringe tip into the adapter opening while pressing and turning firmly in a clockwise direction until the connection is secure.
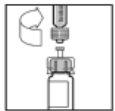
- Slowly push the plunger rod to inject all of the solvent into the ReFacto AF vial.
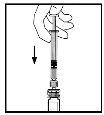
- With the syringe still connected to the adapter, gently swirl the vial until the powder is dissolved.

- Visually inspect the final solution for particles before administration. The solution should appear clear to slightly opalescent and colorless.
Note: If using more than one vial of ReFacto AF per infusion, each vial must be reconstituted following the above instructions. The syringe of solvent should be removed, leaving the vial adapter in place, and another large luer-lock syringe can be used to withdraw the reconstituted contents from each individual vial.
- Make sure the plunger rod of the syringe is still fully inserted, turn the vial upside down, and slowly push the entire solution back into the syringe through the vial adapter.
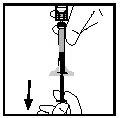
- Separate the syringe from the vial adapter by pulling the syringe and turning it counterclockwise. Discard the vial with the adapter.
Note: If not used immediately, the syringe cap should be carefully replaced. Do not touch the tip of the syringe or the inside of the cap.
ReFacto AF should be used immediately or within 3 hours after reconstitution. Before administration, the reconstituted solution can be stored at room temperature.
Administration (Intravenous Infusion):
ReFacto AF should be administered using the infusion set provided in this package and the pre-filled syringe of solvent or a sterile disposable plastic syringe with a luer-lock connection.
- Attach the syringe to the luer connection of the infusion set.
- Apply a tourniquet and prepare the injection site by cleaning the skin well with one of the provided alcohol swabs.
- Insert the needle of the infusion set into the vein, following your doctor's instructions, and remove the tourniquet. Remove any air that may be in the infusion set by aspirating it into the syringe. The reconstituted product should be injected intravenously over several minutes. Your doctor may adjust the recommended infusion rate to make it more comfortable.
Discard the unused solution, empty vial(s), and used needles and syringes in an appropriate container for disposing of medical waste, as these materials may harm others if not disposed of properly.
If you use more ReFacto AF than you should
Consult your doctor or pharmacist.
If you stop treatment with ReFacto AF
Do not stop using ReFacto AF without consulting your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Allergic reactions
If allergic reactions(anaphylactic) sudden, severeoccur, stop the infusion immediately. Consult your doctor immediatelyif you experience any of the following early symptoms of allergic reactions:
- rash, hives, itching, swelling
- swelling of lips and tongue
- difficulty breathing, wheezing, chest tightness
- general feeling of discomfort
- dizziness and loss of consciousness
Severe symptoms, such as difficulty breathing and fainting (or near-fainting), require rapid emergency treatment. Sudden, severe allergic reactions (anaphylaxis) are uncommon (may affect up to 1 in 100 patients).
Development of inhibitors
In children who have not received prior treatment with factor VIII medicines, inhibitor antibodies may occur very frequently (more than 1 in 10 patients); however, in patients who have received prior treatment with factor VIII (more than 150 days of treatment), the risk is uncommon (less than 1 in 100 patients). If this happens, the medicines you or your child are taking may stop working properly, and you or your child may experience persistent bleeding. In this case, contact your doctor immediately.
Very common side effects(may affect more than 1 in 10 patients)
- development of inhibitors in patients who have never been treated before with factor VIII products
- headache
- cough
- joint pain
- fever
Common side effects(may affect up to 1 in 10 patients)
- bleeding
- dizziness
- loss of appetite, diarrhea, vomiting, stomach pain, nausea
- hives, skin rash, itching
- muscle pain
- chills, catheter site reaction
- some blood tests may show an increase in antibodies against factor VIII
Uncommon side effects(may affect up to 1 in 100 patients)
- development of inhibitors in patients who have been treated before with factor VIII products (less than 1 in 100 patients)
- severe allergic reaction
- numbness, drowsiness, altered taste
- chest pain, rapid heartbeat, palpitations
- low blood pressure, pain and redness of the veins related to the presence of blood clots, flushing
- difficulty breathing
- excessive sweating
- weakness, reaction at the injection site (including pain)
- mild increase in cardiac enzymes
- increase in liver enzymes, increase in bilirubin
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing ReFacto AF
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the outer carton and on the label of the vial after EXP. The expiry date refers to the last day of the month shown.
Store and transport refrigerated (2°C-8°C). Do not freeze to avoid damaging the pre-filled syringe containing the solvent.
For your convenience, the medicine can be removed from the refrigerator and stored at room temperature (up to 25°C) for a single period of up to 3 months. At the end of this room temperature storage period, the product should not be returned to the refrigerator, but should be used or discarded. Record the date you remove ReFacto AF from the refrigerator and store it at room temperature (up to 25°C) on the outer carton. Keep the vial in the outer carton to protect it from light.
The reconstituted product should be used within 3 hours after reconstitution.
The solution will be clear to slightly opalescent and colorless. Do not use this medicine if you notice it is turbid or contains visible particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Container contents and additional information
Composition of ReFacto AF
- The active substance is moroctocog alfa (recombinant coagulation factor VIII). Each vial of ReFacto AF nominally contains 250, 500, 1000 or 2000 IU of moroctocog alfa.
- The other components are sucrose, calcium chloride dihydrate, L-histidine, polysorbate 80, and sodium chloride (see section 2 "ReFacto AF contains sodium"). A solvent [sodium chloride injection solution 9 mg/ml (0.9%)] is also provided for reconstitution.
- After reconstitution with the solvent [sodium chloride solution 9 mg/ml (0.9%)], each vial contains 62.5; 125; 250 or 500 IU, respectively (depending on the potency of moroctocog alfa, i.e., 250, 500, 1000 or 2000 IU) of moroctocog alfa per 1 ml of prepared injection solution.
Appearance of the product and container contents
ReFacto AF is a glass vial with powder for injection and a solvent supplied in a pre-filled syringe.
The container contents are:
- a vial of 250, 500, 1000 or 2000 IU of moroctocog alfa powder
- a pre-filled syringe of solvent, 4 ml of sterile sodium chloride injection solution 9 mg/ml (0.9%), with a plunger rod
- a sterile vial adapter device for reconstitution
- a sterile infusion system
- two alcohol swabs
- adhesive tape
- a gauze
Marketing authorization holder
Pfizer Europe MA EEIG
Boulevard de la Plaine 17
1050 Brussels
Belgium
Manufacturer
Wyeth Farma S.A
Autovía del Norte A-1 Km 23
Desvío Algete Km 1
28700 San Sebastián de los Reyes
Madrid
Spain
For further information about this medicinal product, please contact the local representative of the marketing authorization holder.
Belgium/Belgique/Belgien Luxembourg/Luxemburg Pfizer NV/SA Tel: +32 (0)2 554 62 11 | Lithuania Pfizer Luxembourg SARL filialas Lietuvoje Tel: +370 5 251 4000 |
Bulgaria Pfizer Bulgaria EOOD Pfizer Bulgaria EOOD, 47A Cherni Vrah Blvd. Tel: +359 2 970 4333 | Hungary Pfizer Kft. Tel: +36 1 488 37 00 |
Czech Republic Pfizer, spol. s r.o. Tel: +420 283 004 111 | Malta Vivian Corporation Ltd. Tel: +356 21344610 |
Denmark Pfizer ApS Tlf: +45 44 20 11 00 | Netherlands Pfizer bv Tel: +31 (0)800 63 34 636 |
Germany PFIZER PHARMA GmbH Tel: +49 (0)30 550055-51000 | Norway Pfizer AS Tlf: +47 67 52 61 00 |
Estonia Pfizer Luxembourg SARL Eesti filiaal Tel: +372 666 7500 | Austria Pfizer Corporation Austria Ges.m.b.H. Tel: +43 (0)1 521 15-0 |
Greece Pfizer Ελλάς Α.Ε Tel: +30 210 6785800 | Poland Pfizer Polska Sp. z o.o. Tel: +48 22 335 61 00 |
Spain Pfizer S.L. Tel: +34 91 490 99 00 | Portugal Laboratórios Pfizer, Lda. Tel: +351 21 423 5500 |
France Pfizer Tél: +33 (0)1 58 07 34 40 | Romania Pfizer Romania S.R.L. Tel: +40 (0) 21 207 28 00 |
Croatia Pfizer Croatia d.o.o. Tel: +385 1 3908 777 | Slovenia Pfizer Luxembourg SARL Pfizer, podružnica za svetovanje s podrocja farmacevtske dejavnosti, Ljubljana Tel: +386 (0)1 52 11 400 |
Ireland Pfizer Healthcare Ireland Unlimited Company Tel: 1800 633 363 (toll free) Tel: +44 (0)1304 616161 | Slovakia Pfizer Luxembourg SARL, organizacná zložka Tel: +421 2 3355 5500 |
Iceland Icepharma hf. Tel: +354 540 8000 | Finland Pfizer Oy Tel: +358 (0)9 430 040 |
Italy Pfizer S.r.l. Tel: +39 06 33 18 21 | Sweden Pfizer AB Tel: +46 (0)8 550 520 00 |
Cyprus Pfizer Ελλάς Α.Ε. (Cyprus Branch) Tel: +357 22817690 | |
Latvia Pfizer Luxembourg SARL filiale Latvija Tel: +371 670 35 775 |
Date of last revision of this leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: https://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to REFACTO AF 250 IU, POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 1,000 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1500 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1000 IU - after reconstitution in 2 ml of water for injections, the dose is 500 IU/mlActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription required
Online doctors for REFACTO AF 250 IU, POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about REFACTO AF 250 IU, POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















