
Netenax
Ask a doctor about a prescription for Netenax

How to use Netenax
Package Leaflet: Information for the Patient
Netenax, 3 mg/ml, Eye Drops, Solution in a Single-Dose Container
Netilmicin
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. See section 4.
Package Leaflet Contents
- 1. What is Netenax and what is it used for
- 2. Important information before using Netenax
- 3. How to use Netenax
- 4. Possible side effects
- 5. How to store Netenax
- 6. Contents of the package and other information
1. What is Netenax and what is it used for
Netenax contains the active substance netilmicin, an antibiotic that kills bacteria.
This medicine is indicated for the treatment of superficial eye infections and surrounding areas in adults, caused by bacteria sensitive to netilmicin.
If there is no improvement or the patient feels worse, they should consult a doctor.
2. Important information before using Netenax
When not to use Netenax:
- if the patient is allergic to netilmicin, any other aminoglycoside antibiotic (gentamicin, tobramycin, kanamycin, etc.) or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting to use Netenax, the patient should discuss it with their doctor or pharmacist.
Prolonged use of antibiotics may make the patient more susceptible to eye infections.
Aminoglycoside antibiotics can cause serious hearing and kidney problems. If the patient is using other antibiotics, including oral antibiotics, at the same time as Netenax, they should consult their doctor.
Netenax is not intended for injection and should not be injected into the eye or introduced into the anterior chamber of the eye.
In all of the above cases or in the event of an allergic reaction during use of this medicine, the patient should stop using it and consult a doctor.
Children and adolescents
The safety and efficacy of Netenax in children and adolescents have not been established.
Netenax and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
Netenax can be used at the same time as other eye medicines, but the patient should follow the instructions in section 3.
The patient should tell their doctor or pharmacist if they are taking:
- another antibiotic, in particular polymyxin B, colistin, viomycin, streptomycin, vancomycin, or cephaloridine. Using Netenax with other aminoglycoside antibiotics may increase the risk of kidney or hearing problems or may affect the efficacy of other antibiotics;
- cisplatin (a medicine used to treat cancer);
- diuretics (medicines that help remove excess water from the body) such as ethacrynic acid and furosemide.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before using this medicine.
Pregnancy
The medicine should only be used if the doctor considers it necessary.
Breastfeeding
Using Netenax is not recommended during breastfeeding, as the medicine passes into human milk in small amounts.
Driving and using machines
After using Netenax, temporary blurred vision may occur. The patient should not drive or operate machinery until their vision returns to normal.
3. How to use Netenax
This medicine should always be used exactly as the doctor or pharmacist has told the patient. If the patient is not sure, they should ask their doctor or pharmacist.
The recommended dose is one or two drops into the affected eye, three times a day, or as directed by the doctor.
Contact lenses
The patient should not wear contact lenses during surface treatment of eye infections.
If wearing contact lenses is necessary, using Netenax in single-dose containers is allowed, as it does not contain preservatives.
Instructions for use
- 1) Before instilling the medicine, the patient should wash their hands thoroughly.
- 2) Open the aluminum sachet containing the single-dose containers.
- 3) Check that the single-dose container is intact.
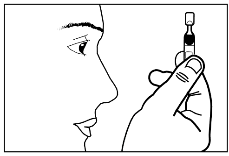
- 4) Detach the single-dose container from the strip and put the unused containers back into the sachet.
- 5) Open the single-dose container by twisting the top, without pulling.
- 6) Do not touch the tip of the container after opening.
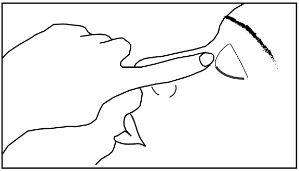
- 7) Gently squeeze the single-dose container to release one drop into the affected eye. Do not touch the tip of the container to the eye, eyelid, or any other surface to avoid contamination.
Without opening the affected eye, press the inner corner of the eye and hold for one minute. This is important, as it reduces the amount of medicine that enters the body.
Using more Netenax than recommended
No cases of overdose with Netenax have been reported.
Missing a dose of Netenax
The patient should not use a double dose to make up for a missed dose.
Stopping use of Netenax
If the patient has any further questions about using this medicine, they should ask their doctor, pharmacist, or nurse.
Using Netenax with other eye medicines
If the patient is using more than one eye medicine, they should wait at least 5 minutes before using the next medicine.
Eye ointments should be used last.
| In the event of accidental overdose, problems are unlikely to occur. The next dose should be used as usual. | |
| The patient should use the next dose at the same time as usual. | |
4. Possible side effects
Like all medicines, Netenax can cause side effects, although not everybody gets them. Most people using these eye drops do not experience any side effects.
The following side effects have been observed during use of Netenax. Frequency not known (frequency cannot be estimated from the available data):
- eye irritation
- eye redness
- eyelid rash
- eyelid swelling
- eye itching
- allergic reaction
- hives
Reporting side effects
If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Medicinal Product Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, the patient can help provide more information on the safety of this medicine.
5. How to store Netenax
The medicine should be stored out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the bottom of each container, on the label, and on the carton after EXP. The expiry date refers to the last day of the month.
Do not store above 25°C.
Single-dose containers should be stored in the original aluminum sachet to protect from light.
The eye drops in single-dose containers are for single use only. After opening the single-dose container, the medicine should be used immediately and the container discarded.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines they no longer need. This will help protect the environment.
6. Contents of the package and other information
What Netenax contains
- The active substance is netilmicin. Each ml of solution contains 3 mg of netilmicin (as netilmicin sulfate).
- The other ingredients are sodium chloride, sodium hydroxide (to adjust pH), purified water.
What Netenax looks like and contents of the pack
Netenax is a clear, colorless or pale yellow solution in single-dose containers made of LDPE containing 0.3 mg/ml eye drops, solution, which are placed in an aluminum sachet in a cardboard box.
Each cardboard box contains 15 or 20 single-dose containers.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
SIFI S.p.A.
Via Ercole Patti 36
95025 Aci S. Antonio (CT)
Italy
[email protected]
This medicine is authorized in the Member States of the European Economic Area under the following names:
Bulgaria, Germany, Latvia, Lithuania: Nettacin
Cyprus, Greece, Poland, Portugal, Slovakia, Spain: Netenax
Czech Republic, France: Anetena
Date of last revision of the package leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterSifi S.p.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NetenaxDosage form: Drops, 3 mg/mlActive substance: netilmicinManufacturer: SIFI SpAPrescription requiredDosage form: Drops, 15 mg/gActive substance: azithromycinManufacturer: Laboratoires Thea Laboratoires UnitherPrescription requiredDosage form: Drops, 15 mg/gActive substance: azithromycinPrescription required
Alternatives to Netenax in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Netenax in Spain
Online doctors for Netenax
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Netenax – subject to medical assessment and local rules.














