
NETENAX 3 mg/ml EYE DROPS SOLUTION IN SINGLE-DOSE CONTAINERS

How to use NETENAX 3 mg/ml EYE DROPS SOLUTION IN SINGLE-DOSE CONTAINERS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Netenax 3mg/ml eye drops solution in single-dose container
netilmicin
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Netenax and what is it used for
- What you need to know before using Netenax
- How to use Netenax
- Possible side effects
- Storage of Netenax
- Contents of the container and additional information
1. What is Netenax and what is it used for
This medication contains the active ingredient netilmicin, an antibiotic belonging to a group called aminoglycosides, which kills bacteria.
Antibiotics are used to treat bacterial infections and are not effective against viral infections. It is essential to follow the instructions regarding dosage, administration interval, and treatment duration as indicated by your doctor. Do not store or reuse this medication. If you have any leftover antibiotic after completing the treatment, return it to the pharmacy for proper disposal. Do not throw away medications down the drain or in the trash. |
It is indicated for adults for the topical treatment of external eye infections and surrounding areas caused by bacteria sensitive to netilmicin.
You should consult a doctor if your condition worsens or does not improve.
2. What you need to know before using Netenax
Do not use Netenax:
- if you are allergic to netilmicin, any other aminoglycoside antibiotic (gentamicin, tobramycin, kanamycin, etc.) or to any of the other components of this medication (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Netenax.
If you use antibiotics for an extended period, you may become more susceptible to eye infections.
Aminoglycoside antibiotics can cause severe ear and kidney problems. If you are using another antibiotic treatment, even orally, along with this medication, consult your doctor.
This medication is not injectable. Therefore, it should not be injected into the eye or introduced into the front part (anterior chamber) of the eye.
In all the above cases, or if you experience allergic reactions with this medication, discontinue use and consult your doctor.
Children and adolescents
The safety and efficacy of this medication have not been established in children or adolescents.
Other medications and Netenax
Inform your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medication.
This medication can be used with other eye medications, but it is essential to follow the instructions in section 3.
Inform your doctor or pharmacist if you are taking:
- any other antibiotic, particularly polymyxin B, colistin, viomycin, streptomycin, vancomycin, or cefaloridine. The simultaneous use of this medication with other aminoglycoside antibiotics may increase the risk of kidney problems, ear problems, or affect the proper functioning of the other antibiotics;
- cisplatin (used in anticancer treatments);
- diuretics (medicines that reduce fluid retention) such as ethacrynic acid and furosemide.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medication.
Pregnancy
You should use this medication only if your doctor considers it appropriate.
Breastfeeding
The use of this medication is not recommended during breastfeeding, as it is excreted in breast milk in small amounts.
Driving and using machines
You may experience temporary blurred vision after using this medication. Do not drive or use machinery until your vision is clear.
3. How to use Netenax
Follow the administration instructions for this medication exactly as indicated by your doctor or pharmacist. If you have any doubts, consult your doctor or pharmacist again.
The recommended dose is 1 or 2 drops in the affected eye 3 times a day or as directed by your doctor.
If you wear contact lenses
The use of contact lenses is not recommended during the treatment of a superficial eye infection.
If you wear contact lenses, use Netenax in a single-dose container, as it does not contain preservatives.
Instructions for use
- Wash your hands thoroughly before applying the eye drops.
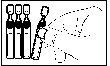
- Open the aluminum pouch containing the single-dose containers.
- Make sure the single-dose container is intact.
- Separate the single-dose container from the strip and put the unopened containers back in the pouch.
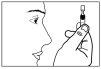
- Open it by twisting the flap without pulling.
- Do not touch the tip after opening the container.

- Gently press the single-dose container to release one drop into the affected eye(s). Do not touch the eye, eyelids, or any other surface with the tip of the bottle to avoid contamination.

Keep the affected eye closed and press the tip of your finger against the tear duct of the closed eye, and hold for 1 minute. This is important because it reduces the amount of medication that passes into the rest of your body.
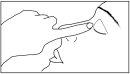
If you use more Netenax than you should
There have been no reported cases of overdose with the use of this medication.
If you accidentally instill more drops than you should, it is unlikely to cause any problems. Apply the next dose as scheduled.
In case of overdose or accidental ingestion, consult your doctor, pharmacist, or call the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount taken.
If you forget to use Netenax
Do not apply a double dose to make up for forgotten doses.
If you stop using Netenax
If you have any further questions about the use of this medication, ask your doctor, pharmacist, or nurse.
If you use Netenax with other eye medications
If you are using any other eye medication, you should wait 5 minutes between using one medication and the other.
Eye ointments should be used last.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone experiences them. Most people treated with this eye drop do not experience any side effects.
The following side effects have occurred with netilmicin. The frequency is unknown (cannot be estimated from the available data):
- eye irritation
- redness in the eyes
- rash on the eyelid
- swelling of the eyelid
- itching in the eyes
- allergic reaction
- hives
Reporting side effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect not listed in this package leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medications: https://www.notificaram.es.By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Netenax
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date shown on the bottom of each unit, on the aluminum pouch, and on the box after CAD. The expiration date is the last day of the month indicated on the unopened and properly stored product.
Do not store above 25°C.
Keep the single-dose containers in the original aluminum pouch to protect them from light.
The eye drops in single-dose containers are for single use. Once the single-dose container is opened, it should be used immediately and the container discarded after use.
Medications should not be thrown away down the drain or in the trash. Deposit the containers and medications you no longer need in the SIGRE collection point at the pharmacy. If you have any doubts, ask your pharmacist how to dispose of the containers and medications you no longer need. This will help protect the environment.
6. Contents of the container and additional information
Composition of Netenax
- The active ingredient is netilmicin. Each ml of solution contains 3 mg of netilmicin (in the form of netilmicin sulfate).
- The other components are sodium chloride, sodium hydroxide (for pH adjustment), and purified water.
Appearance of the product and contents of the container
Netenax is a clear, colorless or pale yellow solution contained in a single-dose container, which is contained in an aluminum pouch that comes in a cardboard box.
Each box contains 15 or 20 single-dose containers.
Only some pack sizes may be marketed.
Marketing authorization holder and manufacturer
SIFI S.p.A.
Via Ercole Patti 36
95025 Aci Sant'Antonio (CT)
Italy
Local representative:
SIFI IBÉRICA S.L.
C/ Poeta Joan Maragall, 47
Planta 4, Puerta 402
28020 Madrid - Spain
Date of the last revision of this package leaflet:November 2019.
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es
- Country of registration
- Average pharmacy price9.3 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NETENAX 3 mg/ml EYE DROPS SOLUTION IN SINGLE-DOSE CONTAINERSDosage form: EYEDROP, 3 mg/mlActive substance: netilmicinManufacturer: Sifi S.P.A.Prescription requiredDosage form: EYEDROP, 3.35 mg azithromycin dihydrateActive substance: azithromycinManufacturer: Laboratoires TheaPrescription requiredDosage form: EYE DROP, 0.3% gentamicinActive substance: gentamicinManufacturer: Ntc S.R.L.Prescription required
Online doctors for NETENAX 3 mg/ml EYE DROPS SOLUTION IN SINGLE-DOSE CONTAINERS
Discuss questions about NETENAX 3 mg/ml EYE DROPS SOLUTION IN SINGLE-DOSE CONTAINERS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















