
OFTALMOWELL EYE DROPS IN SOLUTION

How to use OFTALMOWELL EYE DROPS IN SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
Oftalmowell eye drops in solution
Polymyxin B sulfate, neomycin sulfate, gramicidin
Read the package leaflet carefully before starting to use this medicine because it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet.
Contents of the package leaflet
- What Oftalmowell is and what it is used for
- What you need to know before you use Oftalmowell
- How to use Oftalmowell
- Possible side effects
- Storage of Oftalmowell
- Contents of the pack and further information
1. What Oftalmowell is and what it is used for
Oftalmowell contains several antibiotics (polymyxin B sulfate, neomycin sulfate, and gramicidin) that act against certain microorganisms that cause bacterial infections in the eyes.
Antibiotics are used to treat bacterial infections and are not effective against viral infections.
It is essential to follow the instructions regarding dosage, administration interval, and treatment duration indicated by your doctor.
Do not store or reuse this medicine. If you have any leftover antibiotic after completing the treatment, return it to the pharmacy for proper disposal. Do not throw away medicines via wastewater or household waste.
Oftalmowell is indicated for the treatment of bacterial infections such as purulent bacterial conjunctivitis, keratitis, corneal ulcers, and chronic dacryocystitis.
Oftalmowell is also indicated for ophthalmological prophylaxis before and after surgery, including surgical procedures and removal of foreign bodies from the eye.
2. What you need to know before you use Oftalmowell
Do not use Oftalmowell
- If you are allergic to polymyxin B sulfate, neomycin sulfate, gramicidin, or any of the other components of this medicine (listed in section 6).
- If you are allergic to other related antibiotics, such as framicetin, kanamycin, or gentamicin.
- There is a possibility that absorption may be increased in very young children, so its use is not recommended in neonates or children under 2 years of age. In these populations, absorption through immature skin may increase, and renal function may not be sufficiently developed.
Warnings and precautions
Consult your doctor before starting to use Oftalmowell.
- Use this medicine only in your eye(s).
- Prolonged use of Oftalmowell may produce an overgrowth of non-sensitive microorganisms, including fungi, and the application should be discontinued and appropriate therapy initiated.
- It is not recommended to use contact lenses during the treatment of an eye infection. See section "Oftalmowell contains benzalkonium chloride".
- Cautious use is recommended when used concomitantly with other aminoglycosides or other polypeptide antibiotics systemically.
Using Oftalmowell with other medicines
Tell your doctor or pharmacist if you are using or have recently used other medicines, including those obtained without a prescription.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
This medicine is not recommended during pregnancy.
It is unknown whether the active substances/metabolites of this medicine are excreted in breast milk. A decision must be made whether to discontinue breastfeeding or discontinue treatment, considering the benefit of breastfeeding to the child and the benefit of treatment to the mother.
Driving and using machines
Immediately after applying Oftalmowell, you may notice that your vision becomes blurred. Do not drive or use machines until these effects have disappeared.
Oftalmowell contains benzalkonium chloride
This medicine may cause eye irritation because it contains benzalkonium chloride. Avoid contact with soft contact lenses. Remove contact lenses before application and wait at least 15 minutes before putting them back. It may discolor soft contact lenses.
3. How to use Oftalmowell
Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
The recommended dose is:
Adults (over 18 years):
Purulent bacterial conjunctivitis, keratitis, corneal ulcers, and chronic dacryocystitis
For the first two days, instill one or two drops into the affected eye(s) every 2-4 hours during the day.
Subsequently, instill one or two drops every 4-6 hours until the infection is resolved.
The dosage regimen and treatment duration will be adapted based on medical assessment.
Surgical prophylaxis
Your doctor will establish the dose and administration regimen to be followed both before and after the intervention, as well as in the days following the intervention.
Treatment should continue for at least two days after the problem has been resolved but should not be prolonged for more than 7 days without medical supervision.
Pediatric population:
Oftalmowell eye drops are suitable for use in children (over 2 years) at the same dose as adults. Since there is a probability of increased absorption in very young children, its use is not recommended in neonates and children under 2 years.
Elderly patients:
Oftalmowell is suitable for use in elderly patients. Special caution is required in cases where there is a decrease in renal function, as significant systemic absorption of neomycin sulfate may occur.
Method of administration
Ophthalmic use.
If more than one medicine is used via the ophthalmic route, the applications of the medicines should be spaced at least 5 minutes apart. Ophthalmic ointments should be administered last.
To avoid possible contamination of the dropper tip and the solution, care should be taken not to touch the eyelids, surrounding areas, or other surfaces with the tip of the bottle. The bottle should be kept tightly closed when not in use.
Instructions for use:
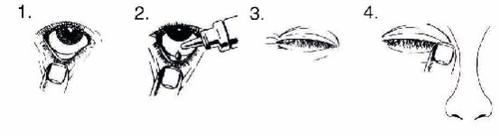
- Wash your hands. Tilt your head back and look up at the ceiling.
- Gently separate the lower eyelid from the eye to form a pouch between the eyelid and your eye, where the drop should fall (figure 1).
- Bring the tip of the bottle close to the eye. You may find it helpful to use a mirror.
- Do not touch the eye or eyelid, surrounding areas, or other surfaces with the bottle. The drops could become contaminated.
- Invert the bottle and gently squeeze it to release one drop at a time into the eye that needs treatment (figure 2). Squeeze gently; do not use force.
- Release the lower eyelid and keep the eye closed for 30 seconds (figure 3).
- After using the eye drops, press the edge of the eye near the nose with your finger. This helps prevent the eye drops from passing into the throat and then into the rest of the body (figure 4).
- If you are applying drops to both eyes, repeat all the previous steps with the other eye. Close the bottle tightly immediately after use.
If a drop falls outside the eye, try again.
If you use more Oftalmowell than you should
If you have applied too much, you can remove it by washing your eyes with lukewarm water. Do not apply more drops until the next dose.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, telephone 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Oftalmowell
If you forget to use Oftalmowell, apply a single dose as soon as you remember, and continue with your usual treatment. Do not apply a double dose to make up for forgotten doses.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following local adverse reactions may occur: burning, irritation, dryness, folliculitis (inflammation of the hair insertion area), hypertrichosis (increased hair growth), eruptions, and striae. These symptoms disappear when the application is discontinued.
The incidence of allergy to neomycin sulfate in the general population is low, but sensitization may occur after its use.
Allergic reactions after topical application of polymyxin B sulfate and gramicidin are rare.
Reporting of side effects:
If you experience any side effects, talk to your doctor or pharmacist, even if they are possible side effects not listed in this package leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Oftalmowell
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton after EXP. The expiry date is the last day of the month stated.
Do not store above 25°C. Store in the original packaging to protect from light.
The bottle should be discarded 4 weeks after the first opening.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicine in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and further information
Composition of Oftalmowell
The active substances are polymyxin B sulfate, neomycin sulfate, and gramicidin. Each ml of sterile solution contains (1 ml = 40 drops): 5,000 IU of polymyxin B sulfate, 1,700 IU of neomycin sulfate, and 25 IU of gramicidin.
The other ingredients are: sodium chloride, ethanol 96%, propylene glycol, poloxamer 188, benzalkonium chloride, sodium hydroxide or diluted sulfuric acid to adjust the pH, and purified water.
Appearance of the product and pack contents
Oftalmowell is an eye drop solution presented in polypropylene bottles with a dropper and screw cap. It contains 5 ml of solution.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Teofarma S.r.l.
Via F.lli Cervi, 8 – 27010
Valle Salimbene (PV) - Italy
Manufacturer: Alcalá Farma S.L.
Avenida de Madrid 82
28802 Alcalá de Henares
Date of the last revision of this package leaflet:July 2013
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price3.89 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OFTALMOWELL EYE DROPS IN SOLUTIONDosage form: EYEDROP, 3.35 mg azithromycin dihydrateActive substance: azithromycinManufacturer: Laboratoires TheaPrescription requiredDosage form: EYE DROP, 0.3% gentamicinActive substance: gentamicinManufacturer: Ntc S.R.L.Prescription requiredDosage form: OPHTHALMIC GEL, 10 MG/GActive substance: fusidic acidManufacturer: Amdipharm LimitedPrescription required
Online doctors for OFTALMOWELL EYE DROPS IN SOLUTION
Discuss questions about OFTALMOWELL EYE DROPS IN SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















