
How to use Mensinorm Set
Leaflet accompanying the packaging: patient information
Mensinorm Set, 75 IU
Mensinorm Set, 150 IU
Powder and solvent for solution for injection
Menotropin
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor orpharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
- This leaflet refers to Mensinorm Set in a dose of75 IU powder and solvent for solution for injection and Mensinorm Set in a dose of150 IU powder and solvent for solution for injection as Mensinorm Set.
Table of contents of the leaflet:
- 1. What is Mensinorm Set and what is it used for
- 2. Important information before using Mensinorm Set
- 3. How to use Mensinorm Set
- 4. Possible side effects
- 5. How to store Mensinorm Set
- 6. Contents of the pack and other information
1. What is Mensinorm Set and what is it used for
- Mensinorm Set is used to stimulate ovulation in women who do not ovulate and have not responded to other treatments (clomiphene citrate).
- Mensinorm Set is used to stimulate the development of multiple follicles (and thus multiple eggs) in women undergoing infertility treatment.
Mensinorm Set is a highly purified human menopausal gonadotropin belonging to a group of
drugs called gonadotropins.
Each vial contains a lyophilized powder containing 75 IU of human follicle-stimulating hormone
(FSH) and 75 IU of human luteinizing hormone (LH).
Human menopausal gonadotropin (HMG) is obtained from the urine of postmenopausal women.
To increase the total LH activity, human chorionic gonadotropin (hCG) has been added — a hormone
obtained from the urine of pregnant women.
Each vial contains a lyophilized powder containing 150 IU of human follicle-stimulating hormone
(FSH) and 150 IU of human luteinizing hormone (LH). Human menopausal gonadotropin (HMG) is
obtained from the urine of postmenopausal women.
To increase the total LH activity, human chorionic gonadotropin (hCG) has been added — a hormone
obtained from the urine of pregnant women.
This medicine should be taken under medical supervision.
2. Important information before using Mensinorm Set
Before starting treatment, an assessment of fertility in both partners will be performed.
Mensinorm Set should not be used if the patient has any of the following conditions:
- hypersensitivity (allergy) to menotropin or any of the other ingredients of Mensinorm Set (listed in section 6)
- enlarged ovaries or ovarian cysts not caused by hormonal disorders (polycystic ovary syndrome);
- bleeding of unknown origin;
- ovarian, uterine, or breast cancer;
- enlargement (tumor) of the pituitary gland or hypothalamus (part of the brain);
This medicine should not be used in women with early menopause, malformation of the reproductive organs, or certain uterine tumors that prevent the development of a normal pregnancy.
Warnings and precautions
So far, no cases of allergic reactions to Mensinorm Set have been reported, however, if the patient has
experienced allergic reactions to similar medicines in the past, they should inform their doctor.
Taking this medicine increases the risk of developing a condition called ovarian hyperstimulation
syndrome (OHSS)(see section 4 Possible side effects). If OHSS occurs, treatment will be discontinued and
conception will not be allowed. The first symptoms of ovarian hyperstimulation are abdominal pain,
nausea, vomiting, and weight gain. If these symptoms occur, the patient should consult their doctor as
soon as possible. In severe, though rare cases, it may lead to ovarian enlargement and fluid accumulation
in the abdominal or chest cavity.
The medicine used to release mature eggs (containing human chorionic gonadotropin, hCG) may
increase the likelihood of OHSS. Therefore, hCG is not recommended in case of developing OHSS.
Sexual intercourse should not be engaged in, even with the use of mechanical contraception, for at least
4 days.
In women with fertility problems, the risk of miscarriage is higher than in the general population.
In women undergoing treatment to induce ovulation, the risk of multiple pregnancies and births is higher
than with natural conception. However, this risk can be reduced by using the recommended dose of the
medicine.
In women with blocked fallopian tubes, there is a slightly increased risk of ectopic pregnancy.
Multiple pregnancy and characteristics of partners undergoing infertility treatment (e.g., woman's age,
sperm parameters) may be associated with an increased risk of congenital anomalies.
Taking Mensinorm Set, like pregnancy itself, may increase the risk of thrombosis. Thrombosis is the formation
of a blood clot in a blood vessel, most often in the veins of the legs or lungs.
Before starting treatment, the patient should discuss this with their doctor, especially in the following
cases:
- the patient has a history of increased risk of thrombosis;
- the patient or their close relatives have a history of thrombosis;
- the patient has massive obesity.
Children
This medicine is not intended for use in children.
Mensinorm Set and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have
recently taken, as well as any medicines they plan to take.
Pregnancy, breastfeeding, and fertility
Mensinorm Set should not be used during pregnancy or breastfeeding.
Driving and using machines
Mensinorm Set has no or negligible influence on the ability to drive and use machines.
Mensinorm Set contains sodium
The medicine contains less than 1 mmol (23 mg) of sodium per prepared solution, i.e., the medicine
is considered "sodium-free".
3. How to use Mensinorm Set
Recommended dose and duration of treatment:
This medicine should always be used as directed by the doctor. In case of doubts, the patient should
consult their doctor.
Women who do not ovulate, with irregular periods or amenorrhea:
Most often, the first injection of the contents of one vial of Mensinorm Set 75 IU is performed during the
first week of the menstrual cycle after a natural or induced period.
Then, Mensinorm Set is administered daily in the dose prescribed by the doctor, and treatment is
continued until at least one mature follicle has developed on the ovary. The doctor will adjust the dose
of Mensinorm Set based on the ovarian response determined by diagnostic tests.
When one follicle has reached the required stage of development, the administration of Mensinorm Set
will be discontinued, and ovulation will be induced using another hormone (human chorionic
gonadotropin, hCG).
Ovulation usually occurs 32-48 hours later.
At this stage of treatment, conception is possible. Sexual intercourse is recommended every day,
starting from the day before hCG administration. If pregnancy does not develop despite ovulation,
treatment can be repeated.
Women undergoing ovarian stimulation to induce the development of multiple follicles before in vitro
fertilization or other assisted reproduction techniques:
The goal of this method is to achieve the simultaneous development of multiple follicles. Treatment
begins on the 2nd or 3rd day of the menstrual cycle with an injection of 150-300 IU of Mensinorm Set
(contents of 1-2 vials of Mensinorm Set 150 IU). The doctor may recommend higher doses of the
medicine if necessary. The dose of Mensinorm Set is higher than that used for natural conception. The
course of further treatment is individualized by the doctor.
When the required number of follicles has reached the required stage of development, the administration
of Mensinorm Set will be discontinued, and ovulation will be induced by an injection of another
hormone (human chorionic gonadotropin, hCG).
How to use Mensinorm Set:
Mensinorm Set is administered as a subcutaneous injection (subcutaneous administration - sc.) or
intramuscular injection (intramuscular administration - im.).
Each vial can only be used once, and the injection should be performed as soon as possible after
preparing the solution for injection.
After proper consultation and training, the doctor may recommend self-administration of Mensinorm
Set injections.
When using the medicine for the first time, the doctor should:
- allow the patient to practice self-administering a subcutaneous injection;
- indicate the areas of the body where the patient can self-administer injections;
- demonstrate how to prepare the solution for injection;
- explain how to prepare the correct dose of the medicine for injection.
Before self-administering a Mensinorm Set injection, the patient should carefully read the following
instructions.
Preparing and injecting the contents of 1 vial of Mensinorm Set:
The solution for injection must be prepared immediately before injection using a pre-filled syringe
with solvent (9 mg/ml sodium chloride solution for injection) provided with each package of
Mensinorm Set.
A clean surface should be prepared, and hands should be washed. It is essential to maintain the highest
possible cleanliness of hands and instruments used.
The following items should be placed on the surface:
- two alcohol swabs (not included in the packaging),
- one vial of Mensinorm Set powder,
- one pre-filled syringe with solvent,
- one needle for preparing the solution for injection,
- one thin needle for subcutaneous injection.
Do not remove the backstop protection (white ring) from the pre-filled syringe, as it prevents accidental
withdrawal of the stopper and facilitates handling of the syringe during injection.
Reconstitution of the solution for injection
Preparing the solution for injection:
- 1.
- Remove the cap from the pre-filled syringe; attach the (long) needle for reconstituting the solution
to the pre-filled syringe. - Carefully place the pre-filled syringe on a clean surface.
- Do not touch the needle.
Preparing the solution for injection:
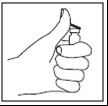
- 2.
- •Remove the colored plastic cap (light green for 75 IU, dark green for 150 IU) from the
Mensinorm Set vial by gently lifting the cap upwards. - Wipe the rubber stopper of the vial with an alcohol swab and wait for it to dry.
- 3.
- Hold the pre-filled syringe, remove the protective cap from the needle, and insert the needle into
the center of the rubber stopper of the Mensinorm Set vial.
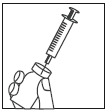
of the rubber stopper of the Mensinorm Set vial.
- Strongly press the plunger of the pre-filled syringe to inject the entire solvent into the powder.
- Do not shake, but gently mix the contents of the vial until a clear solution is obtained. Mensinorm
Set usually dissolves immediately.
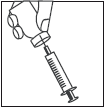
- 4.
- •Without withdrawing the needle from the vial, turn the vial upside down.
- •Make sure the tip of the needle is below the surface of the liquid.
- •Gently pull the plunger to draw the entire Mensinorm Set solution into the syringe.
- •Make sure the solution after reconstitution is clear.
In case of reconstitution of more than one vial of Mensinorm Set, the reconstituted contents of the first
vial should be drawn into the syringe, and then, after repeating steps 2-4, slowly inject it into the
second vial.
Administering a subcutaneous injection of Mensinorm Set:
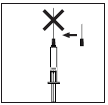
- After drawing the prescribed dose of the medicine into the pre-filled syringe, attach the protective
cap to the needle. Remove the needle from the pre-filled syringe and attach a thin needle for
subcutaneous injection with a protective cap. - Strongly press the thin needle onto the pre-filled syringe and slightly twist to ensure complete and
secure attachment of the needle. - Remove the protective cap from the needle. Holding the pre-filled syringe with the needle facing
upwards, gently tap the side of the pre-filled syringe to make any air bubbles rise to the top of the
pre-filled syringe. - Press the plunger until a drop of liquid appears at the tip of the needle.
- Do not use if the solution contains any solid particles or is cloudy.
Injection site:
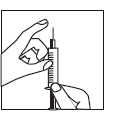
- The patient should have been previously instructed by the doctor or nurse on the selection of the
injection site. The usual injection site is the thigh or the lower abdomen, below the navel. - Wipe the injection site with an alcohol swab.
Inserting the needle:
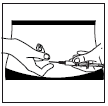
- Firmly grasp a skin fold with the fingers. With the other hand, insert the needle at a 45° or 90° angle
with a quick, decisive motion.
Injecting the solution:
- Inject the solution under the skin, following the instructions. Do not inject the solution directly into
a vein. Press the plunger slowly and at a steady rate, so that the solution is properly injected and
the skin tissues are not damaged.
Inject the prescribed volume of the solution without haste. Depending on the dose prescribed by the
doctor, it may not be necessary to inject the entire volume of the solution.
Withdrawing the needle:
- Quickly withdraw the syringe, pulling out the needle, and then press the injection site with an
alcohol swab. Gentle massage of the injection site while pressing helps to distribute the Mensinorm
Set and reduce discomfort.
Administering an intramuscular injection of Mensinorm Set:
In the case of intramuscular injections, a healthcare professional will prepare Mensinorm Set and then
administer the injection into the patient's thigh or buttock.
Disposal of all used items:
After administering the injection, place all needles, empty vials, and pre-filled syringes in a sharps
container. Any unused solution or waste should be disposed of in accordance with local regulations.
Using a higher dose of Mensinorm Set than recommended:
The effects of overdosing with Mensinorm Set are unknown, but it is likely that ovarian hyperstimulation
syndrome (OHSS) will occur (see section 4 Possible side effects). If a higher dose of Mensinorm Set is
used than recommended, the patient should consult their doctor or nurse.
Missing a dose of Mensinorm Set:
The next dose should be taken at the planned time. Do not take a double dose to make up for a missed
dose.
Stopping treatment with Mensinorm Set:
Do not stop treatment without consulting a doctor. If the patient has any further doubts about
continuing treatment, they should always consult their doctor.
In case of any further doubts about using this medicine, the patient should consult their doctor or
pharmacist.
4. Possible side effects
Like all medicines, Mensinorm Set can cause side effects, although not everybody gets them.
The following side effect is serious and requires immediate action if it occurs. If the patient experiences
the following side effect, they should stop using Mensinorm Set and consult their doctor immediately:
Common, may occur in up to 1 in 10 people:
- Ovarian hyperstimulation syndrome (symptoms include the formation of cysts on the ovaries or
enlargement of existing cysts, abdominal pain, thirst, nausea, vomiting, reduced amounts of
concentrated urine, and weight gain) (see section 2 Important information before using Mensinorm
Set).
The following side effects have also been reported:
Very common (may occur in more than 1 in 10 people):
- headache,
- bloating or swollen abdomen.
Common (may occur in up to 1 in 10 people):
- abdominal pain or discomfort,
- pelvic pain,
- back pain,
- feeling of heaviness,
- breast discomfort,
- dizziness,
- hot flashes,
- thirst,
- nausea,
- fatigue,
- general feeling of being unwell,
- reaction at the injection site, such as pain and inflammation (more common with intramuscular
injection than subcutaneous injection).
Rare (may occur in up to 1 in 1000 people):
- ovarian torsion (twisting of the ovary causing severe abdominal pain).
Very rare (may occur in up to 1 in 10,000 people):
- thromboembolic disease (formation of a blood clot in a blood vessel, which breaks loose and
travels with the blood, and then blocks another blood vessel).
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in the leaflet, they
should tell their doctor or pharmacist. Side effects can be reported directly to the Department of
Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, more information can be gathered on the safety of the medicine.
5. How to store Mensinorm Set
Keep this medicine out of the sight and reach of children.
Do not store above 25°C. Store the vial and pre-filled syringe with solvent in the carton to protect
from light.
Do not use this medicine after the expiry date stated on the outer packaging, vial, and pre-filled syringe
with solvent after: EXP. If the expiry date is stated as a month and year, the expiry date refers to the
last day of the stated month.
Use immediately after reconstitution.
Do not use Mensinorm Set if the solution is cloudy. After reconstitution, the solution should be clear
and colorless.
Medicines should not be disposed of via wastewater. The patient should ask their pharmacist how to
dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Mensinorm Set contains
The active substance is menotropin.
Each vial contains a lyophilized powder containing 75 IU of human follicle-stimulating hormone
(FSH) and 75 IU of human luteinizing hormone (LH).
Human menopausal gonadotropin (HMG) is obtained from the urine of postmenopausal women.
To increase the total LH activity, human chorionic gonadotropin (hCG) has been added — a hormone
obtained from the urine of pregnant women.
Each vial contains a lyophilized powder containing 150 IU of human follicle-stimulating hormone
(FSH) and 150 IU of human luteinizing hormone (LH). Human menopausal gonadotropin (HMG) is
obtained from the urine of postmenopausal women.
To increase the total LH activity, human chorionic gonadotropin (hCG) has been added — a hormone
obtained from the urine of pregnant women.
In the case of using multiple vials of powder, the amount of menotropin contained in 1 ml of the
reconstituted solution will be as follows:
| Mensinorm Set 75 IU powder and solvent for solution for injection | |
| Number of vials used | Total amount of menotropin in 1 ml of solution |
| 1 | 75 IU |
| 2 | 150 IU |
| 3 | 225 IU |
| 4 | 300 IU |
| 5 | 375 IU |
| 6 | 450 IU |
| Mensinorm Set 150 IU powder and solvent for solution for injection | |
| Number of vials used | Total amount of menotropin in 1 ml of solution |
| 1 | 150 IU |
| 2 | 300 IU |
| 3 | 450 IU |
Excipients are:
In the case of powder: lactose monohydrate.
In the case of solvent: 9 mg/ml sodium chloride solution.
What Mensinorm Set looks like and contents of the pack
Powder: white lyophilized disc or powder
Solvent: clear and colorless solution
Mensinorm Set is a powder and solvent for solution for injection.
1 pack contains the following:
- one vial with a white lyophilized disc or powder;
- one pre-filled syringe (1 ml) with a clear, colorless solution;
- one (long) needle for reconstituting the solution and intramuscular injection;
- one (short) needle for subcutaneous injection. Mensinorm Set is available in packs containing 1, 5,
or 10 packs. Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer:
IBSA Farmaceutici Italia srl
Via Martiri di Cefalonia, 2
26900 Lodi – Italy
[email protected]
This medicinal product is authorized in the Member States of the European Economic Area under
the following names:
Austria: Meriofert PFS
Belgium: Fertinorm Kit
Bulgaria: Meriofert PFS
Cyprus: Meriofert PFS
Czech Republic: Meriofert Set
Denmark: Meriofert Set
Estonia: Meriofert Set
Finland: Meriofert Set
France: Fertistartkit
Greece: Meriofert
Hungary: Meriofert Kit
Italy: Meriofert
Lithuania: Meriofert Set
Latvia: Meriofert Set
Luxembourg: Fertinorm Kit
Norway: Meriofert Set
Poland: Mensinorm Set
Romania: Meriofert PFS
Slovakia: Meriofert Kit
Spain: Meriofert Kit
Sweden: Meriofert Set
Netherlands: Meriofert spuit
United Kingdom: Meriofert PFS
Date of last revision of the leaflet:May 2024
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterIBSA Farmaceutici Italia S.r.l.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Mensinorm SetDosage form: Powder, 75 IU FSH + 75 IU LHActive substance: human menopausal gonadotrophinManufacturer: Ferring GmbHPrescription requiredDosage form: Powder, 600 IU FSH + 600 IU LHActive substance: human menopausal gonadotrophinPrescription requiredDosage form: Powder, 1200 IU FSH + 1200 IU LHActive substance: human menopausal gonadotrophinPrescription required
Alternatives to Mensinorm Set in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Mensinorm Set in Spain
Alternative to Mensinorm Set in Ukraine
Online doctors for Mensinorm Set
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Mensinorm Set – subject to medical assessment and local rules.






