
MENOPUR 75 IU POWDER AND SOLVENT FOR INJECTION

How to use MENOPUR 75 IU POWDER AND SOLVENT FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Menopur75 International Units powder and solvent for solution for injection
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. (See section 4).
Contents of the pack
- What is Menopur and what is it used for
- What you need to know before you use Menopur
- How to use Menopur
- Possible side effects
- Storage of Menopur
- Contents of the pack and other information
1. What is Menopur and what is it used for
Menopur comes as a powder that must be mixed with a liquid (solvent) before use. It is given as an injection under the skin or into a muscle.
Menopur contains two hormones called follicle stimulating hormone (FSH) and luteinizing hormone (LH). FSH and LH are natural hormones produced in both men and women. They help the reproductive organs work normally. The FSH and LH in Menopur are obtained from the urine of post-menopausal women. The active ingredient is highly purified and is known as menotropin.
Menopur is used to treat female infertility in the following two situations:
i Women who cannot become pregnant because their ovaries do not produce eggs (including polycystic ovary syndrome). Menopur is used in women who have been treated with a medicine called clomifene citrate to treat their infertility, but this medicine has not worked.
iiWomen in assisted reproduction programs (ART) (including in vitro fertilization/embryo transfer (IVF/ET), gamete intrafallopian transfer (GIFT); and intracytoplasmic sperm injection (ICSI)). Menopur helps the ovaries develop many egg sacs (follicles) where an egg can develop (multiple follicular development).
2. What you need to know before you use Menopur
Before starting treatment with Menopur, you and your partner should be evaluated by a doctor to determine the cause of the infertility problem. In particular, you should be checked for the following diseases so that you can receive the most appropriate treatment:
- Malfunction of the thyroid gland and adrenal cortex
- High levels of a hormone called prolactin (hyperprolactinemia)
- Tumors in the pituitary gland (a gland located at the base of the brain)
- Tumors in the hypothalamus (an area located under the part of the brain called the thalamus).
If you know you have any of the diseases listed above, please inform your doctor before starting treatment with Menopur.
Do not use Menopur:
- If you are allergic (hypersensitive) to Menopur or any of the other ingredients of this medicine (listed in section 6).
- If you have a tumor in the uterus, ovaries, breasts, or certain parts of the brain such as the hypothalamus-pituitary
- If you have fluid-filled sacs (cysts) in the ovaries or enlarged ovaries (unless it is caused by polycystic ovary syndrome)
- If you have malformations of the sex organs or uterus
- If you have vaginal bleeding of unknown cause
- If you have fibroids (benign tumors) in the uterus
- If you are pregnant or breastfeeding
- If you have premature menopause.
Warnings and precautions
Tell your doctor if you have:
- Abdominal pain
- Abdominal swelling
- Nausea
- Vomiting
- Diarrhea
- Weight gain
- Difficulty breathing
- Decreased urine output.
Consult your doctor directly, even if the symptoms develop some days after the last injection was given. These can be signs of high levels of activity in the ovaries that can become serious.
If these symptoms become severe, the fertility treatment should be stopped and you should receive treatment in a hospital.
By maintaining the recommended dose and careful monitoring of the treatment, the likelihood of experiencing these symptoms will be reduced.
If you stop using Menopuryou may still experience these symptoms. Please contact your doctor immediately if you suffer from any of these symptoms.
While you are being treated with this medicine, your doctor will normally perform ultrasound scansand sometimes blood teststo monitor your response to treatment.
When being treated with hormones like Menopur, there is an increased risk of:
- Ectopic pregnancy (pregnancy outside the uterus) if you have a history of disease in the fallopian tubes
- Miscarriage
- Multiple pregnancy (twins, triplets, etc.)
- Congenital malformations (physical defects present in the baby at birth).
Some women who have been treated with several medicines for infertility have developed tumors in the ovaries and other reproductive organs. It is not yet known if treatment with hormones like Menopur causes these problems.
It is more likely that blood clots will form in the blood vessels (veins or arteries) in pregnant women. Fertility treatment may increase the likelihood of this happening, especially if you are overweight or have a known blood clotting disorder (thrombophilia) or if you or a relative have had clotting problems. If you think this might happen to you, inform your doctor.
Use in children
Menopur is not indicated for use in children.
Other medicines and Menopur
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
Clomifene citrate is another medicine used in the treatment of infertility. If Menopur is used at the same time as clomifene citrate, the effect on the ovaries may be increased.
Pregnancy, breastfeeding and fertility
If you are pregnant or breastfeeding, or think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Menopur is not indicated in any case for the treatment of pregnant or breastfeeding women.
Driving and using machines
It is very rare that Menopur affects the ability to drive and use machines.
Important information about some of the ingredients of Menopur
Menopur contains less than 1 mmol of sodium (23 mg) per dose, and is therefore essentially sodium-free.
3. How to use Menopur
Follow the instructions for administration of Menopur exactly as indicated by your doctor.
In case of doubt, consult your doctor or pharmacist again.
- Women who do not ovulate (do not produce eggs):
Treatment will start within the first 7 days of the menstrual cycle (day 1 is the first day of the period). Treatment should be administered every day for at least 7 days.
The initial dose is usually 75-150 IU daily (1-2 vials), which may be adjusted according to the individual patient's response (up to a maximum of 225 IU, 3 vials of powder). This individual dose should be administered for at least 7 days before the dose is adjusted by the doctor. The recommended dose increase is 37.5 IU per adjustment, and should not exceed 75 IU. The treatment cycle should be abandoned if there is no adequate response after 4 weeks.
When an optimal response is obtained, a single injection of another hormone called human chorionic gonadotropin (hCG) will be administered, at a dose of 5,000 to 10,000 IU, one day after the last injection of Menopur. It is recommended that the patient have intercourse on the same day and the day after administration of human chorionic gonadotropin. Intrauterine insemination (injection of sperm directly into the uterus) may be performed alternatively. Your doctor should follow your progress very closely for at least 2 weeks after administration of hCG.
Your doctor will monitor the effect of Menopur treatment. Depending on progress, your doctor will decide to stop treatment with Menopur and not administer the hCG injection. In this case, you should use a contraceptive method (condom) or not have sexual intercourse until the next period begins.
ii. Women in assisted reproduction programs:
If you are also receiving treatment with GnRH agonists, treatment with Menopur should start approximately 2 weeks after starting treatment with GnRH agonists.
If you are also receiving treatment with GnRH antagonists, treatment with Menopur should start on day 2 or 3 of the menstrual cycle (day 1 is the first day of the period).
Menopur will be administered daily for at least 5 days. The recommended initial dose of Menopur is 150-225 IU (2-3 vials of powder).
This dose may be increased according to your response to treatment, up to a maximum of 450 IU (6 vials) per day. The dose should not be increased by more than 150 IU per adjustment. Treatment is not usually recommended for more than 20 days.
If there are enough egg sacs (follicles), a single injection of a medicine called human chorionic gonadotropin (hCG) will be administered at a dose of up to 10,000 IU to induce ovulation (release of an egg).
Your doctor should follow your progress very closely for at least 2 weeks after administration of hCG.
Your doctor will monitor the effect of Menopur treatment. Depending on progress, your doctor will decide to stop treatment with Menopur and not administer the hCG injection. In this case, you should use a contraceptive method (condom) or not have sexual intercourse until the next period begins.
INSTRUCTIONS FOR USE:
If your doctor tells you to inject Menopur yourself, you should follow any instructions that they provide.
The first injection of this medicine should be administered under the supervision of a doctor or nurse.
RECONSTITUTION OF MENOPUR
Menopur is provided as a powder and must be reconstituted before injection. The solvent (liquid) that must be used to reconstitute this medicine is provided with the powder. Menopur should only be reconstituted immediately before use. It should be reconstituted as follows:
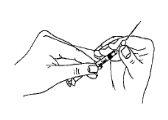 Firmly attach the long, thin needle (preparation/reconstitution needle) to the syringe.
Firmly attach the long, thin needle (preparation/reconstitution needle) to the syringe.
- Open the ampoule of liquid (solvent) with the point facing you.
- Insert the needle into the ampoule of solvent.
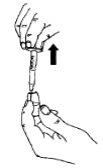 Withdraw all the liquid from the ampoule into the syringe.
Withdraw all the liquid from the ampoule into the syringe.
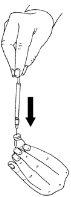
- Insert the needle through the rubber stopper of the vial of powder and slowly inject allthe liquid. Do this on one side of the vial to avoid forming bubbles.
- The powder will dissolve quickly (in 2 minutes) to form a clear solution. Although this usually happens when only a few drops of the solvent have been added, all of the solvent in the ampoule should be added.
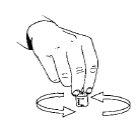
- To help the powder dissolve, gently rotate the solution. Do not shake, as this can cause air bubbles. Do not usethe reconstituted solution if it contains particles or is not clear.
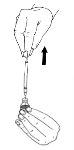
- Withdraw the solution into the syringe.
If you have been prescribed more than one vial of Menopur powder for injection, you may withdraw the solution (the first Menopur solution) back into the syringe and inject it into a second vial of powder. This can be done up to a total of three vials of powder, but only as directed by your doctor.
INJECTION OF MENOPUR
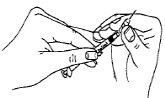
- Once you have your prescribed dose drawn up into the syringe, change the needle (preparation/reconstitution needle) for a short, thin needle (injection needle).
- Hold the syringe upside down and gently tap it to make any air bubbles rise to the top. Slowly push the plunger until all the air has been expelled and a drop of solution appears at the tip of the needle.
- Your doctor or nurse will tell you where to inject (for example, the front of the thigh, abdomen, etc.).
- Disinfect the injection site.
- To inject, pinch the skin to produce a fold, and insert the needle in a quick motion at 90 degrees to the body. Press the plunger to inject the solution, and then withdraw the needle.
- After withdrawing the needle, apply pressure to the injection site to prevent bleeding. Gentle massage at the injection site will help to spread the solution under the skin.
- Do not throw away used materials in the trash; they should be disposed of properly.
If you use more Menopur than you should
In case of overdose or if the solution is swallowed accidentally, consult your doctor or pharmacist immediately or call the Toxicology Information Service, telephone 91 562 04 20, indicating the medicine and the amount used.
If you forget to use Menopur, do not take a double dose to make up for forgotten doses. Please tell your doctor or pharmacist.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Menopur can cause side effects, although not everybody gets them.
Treatment with Menopur may cause high levels of activity in the ovaries, which can lead to a disease called Ovarian Hyperstimulation Syndrome (OHSS), especially in women with polycystic ovaries. The symptoms include: swelling and discomfort in the abdomen, nausea, vomiting, diarrhea, weight gain. In some severe cases of OHSS, the following complications have been reported: accumulation of fluid in the abdomen, pelvis, and/or pleural cavity, difficulty breathing, and decreased urine output. Blood clots in the blood vessels (thromboembolism) and twisting of the ovaries. If you experience any of these symptoms, please contact your doctor immediately, even if they develop some days after the last injection was given.
There may be allergic reactions (hypersensitivity)when using this medicine. The symptoms of these reactions can include: rash, itching, swelling of the throat, and difficulty breathing.If you experience any of these symptoms, contact your doctor immediately.
The following side effects are common (affect 1 to 10 users in 100):
- Abdominal pain
- Headache
- Nausea
- Abdominal swelling (bloating)
- Pelvic pain
- Hyperstimulation of the ovaries resulting in high levels of activity (Ovarian Hyperstimulation Syndrome)
- Local reactions at the injection site (such as pain, redness, bruising, swelling, and/or irritation).
The following side effects are uncommon (affect 1 to 10 users in 1,000):
- Vomiting
- Abdominal discomfort
- Diarrhea
- Fatigue
- Dizziness
- Fluid-filled sacs in the ovaries (ovarian cysts)
- Breast complications (including breast pain, breast tension, breast discomfort, nipple pain, and breast swelling)
- Hot flashes
The following side effects are rare (affect 1 to 10 users in 10,000):
- Acne
- Rash
In addition to the side effects listed above, the following side effects have been reported after marketing of Menopur, with an unknown frequency:
- Visual disturbances
- Fever
- Feeling unwell
- Allergic reactions
- Weight gain
- Pain in the muscles and joints (e.g. back pain, neck pain, arm pain, and leg pain)
- Torsion of the ovaries as a complication of increased ovarian activity due to hyperstimulation.
- Itching
- Hives
- Blood clots as a complication of increased ovarian activity due to hyperstimulation.
If you experience any side effect, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet.
Reporting of side effects.
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Menopur
Do not store above 25 ° C. Do not freeze.
Keep in the outer packaging to protect from light.
For immediate and single use after reconstitution.
Keep out of sight and reach of children.
Do not use Menotropina Ferring after the expiration date shown on the box after CAD. The expiration date is the last day of the month indicated.
Medicines should not be disposed of through wastewater or household waste. Deposit the packaging and medicines you no longer need at the pharmacy's SIGRE Point. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package contents and additional information
Composition ofMenopur
The active ingredient is highly purified menotropin (human menopausal gonadotropin, hMG) which corresponds to 75 IU of follicle-stimulating hormone (FSH) activity and 75 IU of luteinizing hormone (LH) activity.
The other components of the powder are: lactose monohydrate, polysorbate 20, sodium hydroxide, and hydrochloric acid.
The components of the solvent are: sodium chloride, diluted hydrochloric acid, sodium hydroxide, and water for injectable preparations.
Appearance of the product and package contents
Menopur is a powder and solvent for injectable solution.
The package contains five or ten glass vials of transparent glass containing a lyophilized, white to grayish compact powder. The package also contains an equal number of glass ampoules of transparent glass containing the solvent, a clear, colorless solution, for reconstitution.
Only some package sizes may be marketed.
Marketing Authorization Holder and manufacturer:
Marketing Authorization Holder
Ferring, S.A.U.
C/ del Arquitecto Sánchez Arcas nº3, 1º
28040 Madrid
Spain
Manufacturer:
FERRING GmbH
Wittland 11,
D-24109 Kiel
Germany
This medicinal product is authorized in the Member States of the European Economic Areawith the following names:
Belgium, Ireland, Luxembourg, Slovakia: Menotrophin Ferring
Czech Republic: Menotrophin Ferring-Léciva
Bulgaria, Croatia, Cyprus, Denmark, Estonia, Finland, Germany, Greece, Hungary, Iceland, Latvia, Lithuania, Malta, Norway, Portugal, Romania, Slovenia, Spain, Sweden: Menopur
Italy: Meropur
Date of the last revision of this leafletMay 2025.
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MENOPUR 75 IU POWDER AND SOLVENT FOR INJECTIONDosage form: INJECTABLE, -Active substance: human menopausal gonadotrophinManufacturer: Angelini Pharma Espana S.L.Prescription requiredDosage form: INJECTABLE, 1200 IUActive substance: human menopausal gonadotrophinManufacturer: Ferring S.A.Prescription requiredDosage form: INJECTABLE, 1200 IUActive substance: human menopausal gonadotrophinManufacturer: Ferring S.A.U.Prescription required
Online doctors for MENOPUR 75 IU POWDER AND SOLVENT FOR INJECTION
Discuss questions about MENOPUR 75 IU POWDER AND SOLVENT FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






