
How to use Menopur
PATIENT INFORMATION LEAFLET
Leaflet attached to the packaging: patient information
MENOPUR, 1200 IU FSH + 1200 IU LH, powder and solvent for solution for
injection
Menotropin
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor. See section 4.
Table of contents of the leaflet:
- 1. What is Menopur and what is it used for
- 2. Important information before using Menopur
- 3. How to use Menopur
- 4. Possible side effects
- 5. How to store Menopur
- 6. Contents of the pack and other information
1. What is Menopur and what is it used for
Menopur is a powder (in this case, a solid, compact mass) that must be dissolved in a liquid (solvent) before use. The medicine is given as a subcutaneous or intramuscular injection. Menopur (highly purified menotropin obtained from the urine of postmenopausal women) contains two hormones: follicle-stimulating hormone (FSH) and luteinizing hormone (LH). FSH and LH are natural hormones produced in the body of a woman and a man. They enable the normal functioning of the reproductive organs. Menopur is used to treat infertility in the following situations: in women who cannot become pregnant because their ovaries do not produce egg cells (also in the case of so-called polycystic ovary syndrome). Menopur is used in women who have already been treated with clomiphene citrate for infertility, but the medicine has been ineffective; in women participating in assisted reproduction programs, such as in vitro fertilization and embryo transfer, gamete intrafallopian transfer, and intracytoplasmic sperm injection. Menopur supports the production of multiple ovarian follicles by the ovaries, in which egg cells can develop (development of multiple ovarian follicles); in men whose testes produce insufficient sperm due to a lack of gonadotropins (hormones produced by the pituitary gland and affecting the testes).
2. Important information before using Menopur
Before starting treatment with Menopur, it is necessary for the doctor to assess the causes of fertility disorders in both partners. In particular, it should be checked whether the following diseases are present, which require different, appropriate treatment: hypothyroidism and adrenal insufficiency high levels of a hormone called prolactin (hyperprolactinemia) pituitary tumors (a gland located at the base of the brain) hypothalamic tumors (an area located under the part of the brain called the hypothalamus). If the patient has been diagnosed with any of the above diseases, the doctor should be informed before starting treatment with Menopur.
When not to use Menopur
In women and men: if the patient is allergic to menotropin or any of the other ingredients of this medicine (listed in section 6); if pituitary or hypothalamic tumors have been diagnosed. In women: if the patient is pregnant or breastfeeding; if the patient has ovarian cysts or ovarian enlargement not caused by polycystic ovary syndrome; if the patient has vaginal bleeding of unknown cause; if the patient has tumors of the uterus, ovaries, or breasts; if the patient has developmental abnormalities of the genital organs that prevent pregnancy; if the patient has uterine fibroids that prevent pregnancy; if the patient has premature menopause. In men: if the patient has prostate cancer; if the patient has testicular tumors.
Warnings and precautions
Caution should be exercised if the patient experiences: abdominal pain; abdominal distension; nausea; vomiting; diarrhea; weight gain; breathing difficulties; decreased frequency or amount of urine passed. The above symptoms should be reported to the doctor immediately, even if they occur several days after the last dose of the medicine. They may be symptoms of excessive ovarian activity, which can be severe. If these symptoms worsen, fertility treatment should be discontinued and appropriate treatment initiated in the hospital. Adherence to the prescribed dose and careful monitoring of the treatment course reduces the likelihood of these symptoms. These symptoms may also occur when Menopur is discontinued. If any of these symptoms occur, the doctor should be contacted immediately. During treatment with Menopur, the doctor usually refers the patient for ultrasound examinations (using ultrasound) and sometimes for blood tests to check the response to treatment. Hormone treatment, such as Menopur, may increase the risk of: ectopic pregnancy (outside the uterus) in women with previously diagnosed tubal diseases; miscarriage; multiple pregnancy (twins, triplets, etc.); congenital malformations (physical defects present at birth). In some women treated for infertility, ovarian tumors or other reproductive organ tumors have developed. It is not yet known whether this was caused by hormone treatment such as Menopur. The risk of blood clots in veins or arteries is higher in pregnant women. Fertility treatment may increase the risk of blood clots, especially if the patient is overweight or if blood clots have occurred previously in the patient or in their family (relatives). The patient should inform the doctor if they think this applies to them. Menopur, in combination with a hormone called human chorionic gonadotropin (hCG), may be administered in the treatment of male infertility.
Children and adolescents
The use of Menopur in children and adolescents is not appropriate.
Menopur and other medicines
Tell your doctor about all medicines you are taking, or have recently taken, and about medicines you plan to take. Clomiphene citrate is another medicine used to treat infertility. If Menopur is given at the same time as clomiphene citrate, the effect on the ovaries may be enhanced. Menopur can be used in combination with Bravelle. See section 3.
Pregnancy and breastfeeding
Menopur should not be used during pregnancy or breastfeeding.
Driving and using machines
It is unlikely that Menopur will affect the ability to drive and use machines.
Menopur contains sodium
Menopur contains less than 1 mmol of sodium (23 mg) per dose, i.e., the medicine is considered "sodium-free".
3. How to use Menopur
This medicine should always be used as directed by your doctor. In case of doubt, consult your doctor. Women who do not ovulate (do not produce egg cells) Treatment should start within the first 7 days of the menstrual cycle (day 1 is the first day of menstruation). The medicine should be given daily for at least 7 days. The initial dose is usually 75 IU (International Units) FSH + 75 IU LH to 150 IU FSH + 150 IU LH per day. Depending on the patient's response, the dose can be increased to a maximum of 225 IU FSH + 225 IU LH. The prescribed dose should be given for at least 7 days before the dose is changed. It is recommended to increase the dose by 37.5 IU FSH + 37.5 IU LH at each change, but not more than 75 IU FSH + 75 IU LH. The treatment cycle should be discontinued if no response is observed after 4 weeks. When a satisfactory response is achieved, the next day after the last injection of Menopur, another hormone - hCG should be given in a single injection at a dose of 5000 IU to 10,000 IU. It is recommended to have sexual intercourse on the day of administration and the next day after administration of hCG. Alternatively, insemination (insertion of semen directly into the uterus) can be performed. The patient remains under close supervision of the doctor for at least 2 weeks after administration of hCG. The doctor will monitor the results of Menopur treatment. Depending on the progress of treatment, the doctor may decide to discontinue Menopur administration and refrain from administering hCG. In such a case, the patient will be instructed to use a mechanical contraceptive method (e.g., condom) or abstain from sexual intercourse until the next menstruation. Women participating in assisted reproduction programs If the patient has also been treated with a GnRH agonist (a medicine that supports the action of gonadotropin-releasing hormone), Menopur administration should be started about 2 weeks after the start of GnRH agonist treatment. If the patient has also been treated with a GnRH antagonist, Menopur administration should be started on the 2nd or 3rd day of the menstrual cycle (1st day of menstruation is the 1st day of the cycle). The medicine should be given daily for at least 5 days. The initial dose of Menopur is usually 150 IU FSH + 150 IU LH to 225 IU FSH + 225 IU LH per day. Depending on the patient's response to treatment, this dose may be increased to a maximum of 450 IU FSH + 450 IU LH per day. The dose should not be increased by more than 150 IU FSH + 150 IU LH at a time. Treatment usually should not last longer than 20 days. After a sufficient number of appropriately sized ovarian follicles has been observed, the patient receives a single injection of hCG at a dose of up to 10,000 IU to induce ovulation (release of an egg cell). The patient remains under close supervision of the doctor for at least 2 weeks after administration of hCG. The doctor will monitor the results of Menopur treatment. Depending on the progress of treatment, the doctor may decide to discontinue Menopur administration and refrain from administering hCG. In such a case, the patient will be instructed to use a mechanical contraceptive method (e.g., condom) or abstain from sexual intercourse until the next menstruation. Men: Treatment starts with the administration of hCG 3 times a week at a dose of 1000 IU to 3000 IU until a normal testosterone level is achieved in the blood serum. Then, Menopur is given intramuscularly at a dose of 75 IU FSH + 75 IU LH to 150 IU FSH + 150 IU LH 3 times a week for several months.
INSTRUCTIONS FOR USE
If the doctor has recommended self-injection of Menopur, all instructions provided should be followed. The first injection of Menopur should be performed under the supervision of a doctor. Menopur is a powder in a vial and must be reconstituted (dissolved) before injection. The liquid used to dissolve Menopur is in two ampoules provided with the vial of powder.
Menopur 1200 IU FSH + 1200 IU LH must be dissolved in the solvent contained in the two ampoules before use.
After dissolution, the medicine in one vial is given over several days, and therefore, it should be ensured that only the dose prescribed by the doctor is taken. The doctor prescribes the dose of Menopur in International Units (IU). One of the 18 syringes for injection, calibrated in International Units of FSH/LH, which are in the packaging, should be used.

To do this:
1  | 2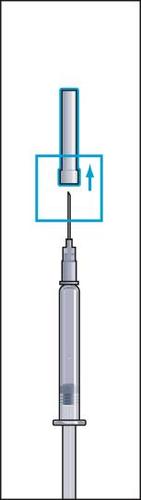 |
- 1. Remove the protective cap from the vial of powder and the rubber cover from one of the ampoule syringes with solvent (Figure 1).
- 2. Attach the needle (reconstitution needle) firmly to the ampoule syringe with solvent and remove the protective cover from the needle (Figure 2).
3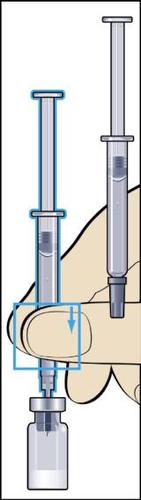 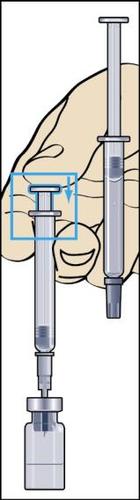 | 4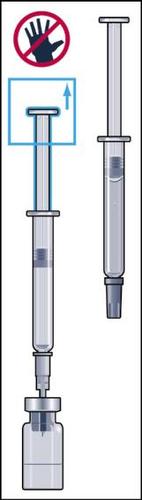 | 5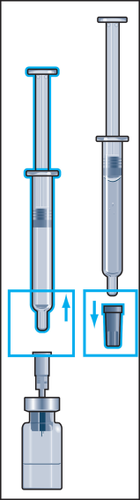 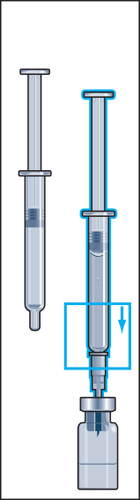 | 6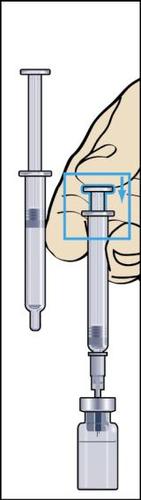 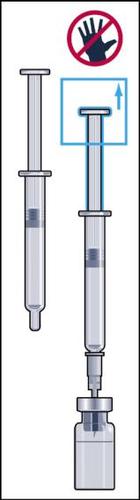 |
- 3. Insert the needle vertically through the center of the rubber stopper of the vial of powder and slowly inject all the liquid into the vial to avoid creating air bubbles (Figure 3).
- 4. During injection of the solvent into the vial, overpressure is created. Therefore, after injection of the solvent, the pressure on the plunger should be released for about 10 seconds and allowed to return to the top. This will eliminate the overpressure in the vial (Figure 4).
- 5. Gently rotate to detach the ampoule syringe from the needle, leaving the needle in the vial. Remove the rubber cover from the second ampoule syringe with solvent and firmly attach the ampoule syringe to the needle stuck in the vial. Slowly inject all the liquid into the vial to avoid creating air bubbles (Figure 5).
- 6. During injection of the solvent into the vial, overpressure is created. Therefore, after injection of the solvent, the pressure on the plunger should be released for about 10 seconds and allowed to return to the top. This will eliminate the overpressure in the vial (Figure 6).
Discard the ampoule syringes and reconstitution needle.
7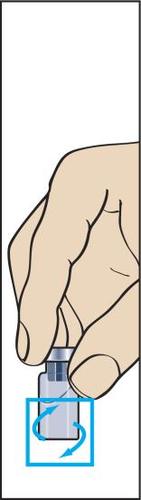 | 8 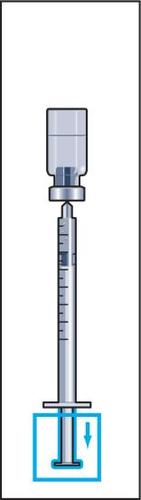 | 9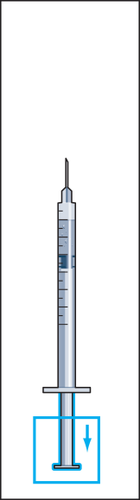 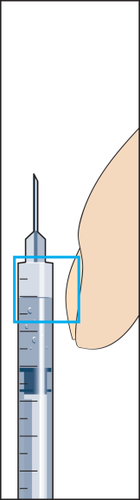 | 10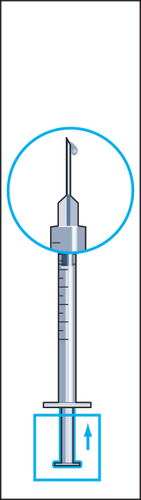 |
- 7. The powder should dissolve quickly (within 2 minutes), forming a clear solution. Although it usually happens after injecting a few drops of solvent, the entire solvent should be injected. To facilitate dissolution of the powder, the vial can be rocked (Figure 7). The vial should not be shaken, as this causes air bubbles to form.
If the solution is not clear or contains solid particles, it should not be used.
The solution formed after dissolving the powder in the solvent from the two ampoule syringes is now ready for use.
- 8. Take a syringe for injection with a attached needle out of the packaging and insert the needle vertically through the center of the rubber stopper of the vial. The syringe for injection contains a small amount of air, which should be injected into the vial above the solution. Invert the vial and draw the prescribed dose of Menopur into the syringe for injection (Figure 8).
NOTE: since the vial contains medicine intended for use over several days, ensure that only the dose prescribed by the doctor is taken.
If the doctor has prescribed Bravelle with Menopur, the two medicines can be mixed. To do this, after reconstituting Menopur, the prescribed dose of Menopur should be injected into the vial with the reconstituted solution of Bravelle. Then, the mixed solution containing both medicines should be drawn into the syringe and injected subcutaneously. This procedure allows avoiding injection of each medicine separately.
- 9. Detach the syringe from the vial and draw a small amount of air into the syringe (Figure 9).
- 10. Hold the syringe with the needle upwards and gently tap the syringe for injection with your finger so that all air bubbles collect at the top (Figure 10). Remove the air from the syringe by pressing the plunger gently until the first drop of liquid appears at the tip of the needle.
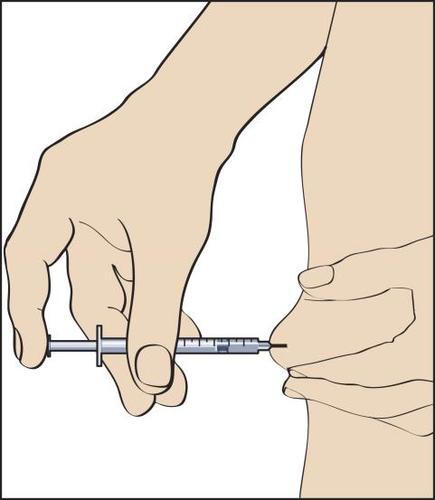
The patient is informed by the doctor or nurse where to inject the medicine (e.g., front of the thigh, abdomen, etc.). Before injection, the skin at the injection site should be disinfected.
11
- 11. To perform the injection, a skin fold should be formed between the fingers and the needle inserted quickly at a 90-degree angle (perpendicularly). Inject the solution by gently pressing the plunger of the syringe (Figure 11), and then withdraw the syringe with the needle.
After withdrawing the syringe with the needle, press the injection site to prevent any bleeding. Gentle massage at the injection site facilitates the spread of the solution under the skin. Used items should not be thrown into household waste or domestic waste containers, but disposed of in an appropriate manner.
- 12. Subsequent injections of the reconstituted Menopur solution are performed by repeating the steps described in points 8 to 11.
Using a higher dose of Menopur than recommended
In case of using a higher dose of Menopur than recommended, the doctor should be informed.
Missing a dose of Menopur
A double dose should not be used to make up for a missed dose. In case of missing a dose of Menopur, the doctor should be informed.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. Hormones used to treat infertility, such as Menopur, can cause excessive ovarian activity, leading to a condition called ovarian hyperstimulation syndrome (OHSS), especially in women with polycystic ovary syndrome. Symptoms include: abdominal pain; abdominal distension; nausea; vomiting; diarrhea; weight gain; and decreased frequency or amount of urine passed. In severe cases of OHSS, rare complications have occurred, such as fluid accumulation in the abdominal cavity, pelvic cavity, and (or) pleural cavity, breathing difficulties, and decreased frequency or amount of urine passed, as well as the formation of blood clots in blood vessels (thromboembolic disorders) and twisting of the ovaries. If any of these symptoms occur, the doctor should be contacted immediately, even if they occur several days after the last dose of the medicine. During treatment with Menopur, allergic reactions (hypersensitivity) may occur. Symptoms of such reactions may include: rash; itching; swelling of the throat; and breathing difficulties. If any of these symptoms occur, the doctor should be contacted immediately. The following side effects are common(affecting 1 to 10 in every 100 patients): abdominal pain; headache; nausea; abdominal distension; pelvic pain; excessive ovarian stimulation causing high activity (ovarian hyperstimulation syndrome); reactions at the injection site, such as pain, redness, bruising, swelling, and (or) itching. The following side effects are uncommon(affecting 1 to 10 in every 1,000 patients): vomiting; abdominal complaints; diarrhea; fatigue; dizziness; ovarian cysts; breast disorders, including breast pain, breast tenderness, discomfort, nipple pain, and breast swelling; hot flashes. The following side effects are rare(affecting 1 to 10 in every 10,000 patients): acne; rash. In addition to the above, the following side effects have been observed after Menopur was placed on the market, and their frequency is unknown: visual disturbances; fever; malaise; allergic reactions; weight gain; muscle and joint pain (e.g., back pain, neck pain, and pain in the arms and legs); ovarian torsion, as a complication of increased ovarian activity caused by excessive stimulation; itching; hives; blood clots, as a complication of increased ovarian activity caused by excessive stimulation.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, please tell your doctor. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Aleje Jerozolimskie 181C, 02-222 Warsaw Tel: +48 22 49 21 301 Fax: +48 22 49 21 309 e-mail: [email protected] Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Menopur
Keep this medicine out of the sight and reach of children. Before reconstitution, store in a refrigerator (2°C - 8°C). Do not freeze. Store in the original packaging to protect from light. After reconstitution, the solution can be stored for 28 days at a temperature below 25°C. Do not freeze. Do not use the solution if it contains solid particles or is not clear. Do not use this medicine after the expiry date stated on the packaging after EXP. The expiry date refers to the last day of the month stated. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Menopur contains
- The active substance of Menopur is highly purified menotropin (Menotropinum) (human menopausal gonadotropin, hMG) in an amount equivalent to 1200 IU FSH (follicle-stimulating hormone) and 1200 IU LH (luteinizing hormone).
- The other ingredients are: Powder: lactose monohydrate, polysorbate 20, disodium phosphate heptahydrate, phosphoric acid (concentrated) Solvent: water for injection, metacresol (preservative)
What Menopur looks like and contents of the pack
Menopur is a powder and solvent for solution for injection. The pack contains: 1 vial of powder; 2 ampoule syringes with solvent for reconstitution; 1 reconstitution needle; 18 single-use syringes for injection calibrated in FSH/LH units with attached needles.
Marketing authorization holder and manufacturer:
Ferring GmbH Wittland 11, D-24109 Kiel, Germany Date of last revision of the leaflet:01/2018 For further information about this medicine, please contact the representative of the marketing authorization holder: Ferring Pharmaceuticals Poland Sp. z o.o. ul. Szamocka 8, 01-748 Warsaw Tel.: +48 22 246 06 80, Fax: +48 22 246 06 81
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterFerring GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MenopurDosage form: Powder, 75 IU FSH + 75 IU LHActive substance: human menopausal gonadotrophinManufacturer: Ferring GmbHPrescription requiredDosage form: Powder, 600 IU FSH + 600 IU LHActive substance: human menopausal gonadotrophinPrescription requiredDosage form: Powder, 150 IU FSH + 150 IU LHActive substance: human menopausal gonadotrophinManufacturer: Ferring GmbHPrescription required
Alternatives to Menopur in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Menopur in Hiszpania
Alternative to Menopur in Ukraina
Online doctors for Menopur
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Menopur – subject to medical assessment and local rules.






