
MENOPUR 1200 IU PRE-FILLED PEN SOLUTION FOR INJECTION

How to use MENOPUR 1200 IU PRE-FILLED PEN SOLUTION FOR INJECTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Menopur1200 IU solution for injection in pre-filled pen
Menotropin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Menopur and what is it used for
- What you need to know before you use Menopur
- How to use Menopur
- Possible side effects
- Storage of Menopur
- Contents of the pack and other information
1. What is Menopur and what is it used for
Menopur is presented as a solution for injection in a pre-filled pen. The injection is given under the skin (subcutaneous injection), usually in the abdomen.
Menopur contains menotropin which is a mixture of two natural hormones called:
- follicle stimulating hormone (FSH) and
- luteinizing hormone (LH).
These hormones help the reproductive organs to function normally. The FSH and LH hormones in menotropin are obtained from the urine of post-menopausal women.
What is Menopur used for
Menopur is used to treat women who cannot become pregnant. It is used for:
- Women whose ovaries do not produce eggs. This includes women with polycystic ovary syndrome (PCOS). Menopur is used in women who have been treated with clomifene citrate but this medicine has not worked.
- Women in assisted reproduction programs (ART). This includes:
- in vitro fertilization/embryo transfer (IVF/ET),
- gamete intrafallopian transfer (GIFT),
- intracytoplasmic sperm injection (ICSI).
How Menopur works
Menopur helps the ovaries to develop many egg sacs (follicles) where an egg can develop. This is called "multiple follicular development".
2. What you need to know before you use Menopur
Checks before using Menopur
Before starting treatment with Menotropin, you and your partner should be evaluated by a doctor to determine the cause of the infertility problem. In particular, you should be checked for the following diseases so that you can receive the most appropriate treatment:
- Malfunction of the thyroid gland and adrenal cortex
- High levels of a hormone called prolactin (hyperprolactinemia)
- Tumors in the pituitary gland (a gland located at the base of the brain)
- Tumors in the hypothalamus (an area located under the part of the brain called the thalamus).
If you know you have any of the diseases listed above, please inform your doctor before starting treatment with Menopur.
Do not use Menopur if:
- you are allergic (hypersensitive) to menotropin or any of the other components of Menopur listed in section 6.
- you have a tumor in the uterus, ovaries, breasts, or some parts of the brain such as the hypothalamus-pituitary
- you have enlarged ovaries or cysts not caused by polycystic ovary syndrome
- you have malformations of the sexual organs or uterus
- you have vaginal bleeding of unknown cause
- you have fibroids (benign tumors) in the uterus
- you are pregnant or breastfeeding
Warnings and precautions
Ovarian Hyperstimulation Syndrome (OHSS)
A serious side effect of this medicine, especially in women with PCOS, is "ovarian hyperstimulation syndrome" or "OHSS" (see section 4).
Tell your doctor immediately if you have signs of OHSS, even if:
- it has been a few days since your last injection
- you stop using Menopur.
These can be signs of high levels of activity in the ovaries, which can become serious. If this happens, your doctor will stop your treatment with Menopur and treat you in a hospital.
Maintaining the recommended dose and careful monitoring of treatment will reduce the options for suffering from these symptoms.
Scans and tests
While you are being treated with this medicine, your doctor will normally send you for ultrasound scansand sometimes blood teststo monitor your response to treatment.
Pregnancy risks
When you are being treated with hormones like Menopur, the risk of:
- ectopic pregnancy (pregnancy outside the uterus) if you have a history of fallopian tube disease
- miscarriage
- multiple pregnancy (twins, triplets, etc.)
- congenital malformations (physical defects present in the baby at birth).
Some women who have been treated with several medications for infertility have developed tumors in the ovaries and other reproductive organs. It is not yet known if treatment with hormones like this medicine causes these problems.
Blood clots
It is more likely that blood clots will form in the blood vessels (veins or arteries) in pregnant women. Infertility treatment may increase the likelihood of this happening, especially if you:
- are overweight
- have a known blood clotting disorder (thrombophilia)
- you or a relative have had blood clotting problems.
Tell your doctor if you think this applies to you.
Use in children
Menotropin is not indicated for use in children.
Other medicines and Menopur
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
Clomifene citrate is another medicine used in the treatment of infertility. If menotropin is used at the same time as clomifene citrate, the effect on the ovaries may be increased.
Pregnancy, breastfeeding and fertility
If you are pregnant or breastfeeding, or think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Do not use Menopur if you are pregnant or breastfeeding.
Driving and using machines
It is very rare that Menopur affects the ability to drive and use machines.
Important information about some of the ingredients of Menopur
This medicine contains less than 1 mmol of sodium (23 mg) per dose unit; this is essentially "sodium-free".
3. How to use Menopur
Follow the instructions for administration of Menopur exactly as indicated by your doctor. If you are in doubt, consult your doctor or pharmacist again.
Women who do not ovulate (do not produce eggs):
Your treatment will start within the first 7 days of your menstrual cycle:
- day 1 is the first day of your period
- . Treatment should be administered every day for at least 7 days.
How much Menopur?
The initial dose is usually 75-150 IU per day.
- This dose may be increased according to your response to treatment, up to a maximum of 225 IU per day.
- You will have the chosen dose for at least 7 days before your doctor changes it.
- . The dose will normally be increased by 37.5 IU per adjustment. It will not be increased by more than 75 IU.
Your doctor will monitor the effect of treatment with Menopur. The treatment cycle should be abandoned if you do not respond adequately after 4 weeks.
If you have a good response to Menopur
You will be given a single injection of a hormone called human chorionic gonadotropin (hCG).
- The dose will be between 5,000 and 10,000 IU
- You will receive the hCG injection 1 day after the last dose of Menopur.
It is recommended to have intercourse on the same day as the administration of hCG andthe next day. Alternatively, sperm can be injected directly into the uterus, known as artificial insemination.
- Your doctor should follow your progress very closely for at least 2 weeks.
If you do not respond to Menopur:
- Your doctor will follow the effect of Menopur treatment.
- Depending on your progress, your doctor may decide to interrupt treatment with Menopur and not administer the hCG injection.
Women in assisted reproduction programs:
If you are in an assisted reproduction program, you will also have a medicine that helps a hormone called "gonadotropin-releasing hormone" (GnRH) to work. This other medicine is called a "GnRH agonist". Menopur should be started around 2 weeks after the start of therapy with GnRH agonists.
You may also be taking a medicine called a "GnRH antagonist".
Treatment with Menopur should be started on day 2 or 3 of the menstrual cycle (day 1 is the first day of your period).
How much Menopur?
Menopur should be administered every day for at least 5 days.
- The initial dose of Menopur is usually 150 to 225 IU.
- This dose may be increased according to your response to treatment, up to a maximum of 450 IU per day.
- The dose should not be increased by more than 150 IU at a time.
Normally, treatment should not continue for more than 20 days.
If there are enough egg sacs (or follicles), you will be given a single injection of hCG in a dose of up to 10,000 IU to trigger the release of an egg (ovulation).
Your doctor will closely monitor your progress for at least 2 weeks after you have been given the hCG injection.
Your doctor will monitor the effect of treatment with Menopur.
- Depending on your progress, your doctor may decide to interrupt treatment with Menopur and not administer the hCG injection.
- In this case, you will be advised to use a barrier contraceptive method (e.g. a condom).
Otherwise, you should not have sexual intercourse until you have started your next period.
Using Menopur
Follow the "Instructions for use" carefully, which are supplied with the pre-filled pen.
A doctor or nurse will be present for your first injection of Menopur. Your doctor will decide if you can administer the following injections yourself at home, after you have received complete training.
Menopur will be administered as an injection under the skin (subcutaneous injection). This is usually in the abdomen. Each pre-filled pen can be used for several injections.
If you use more Menopur than you should.In case of overdose or if the solution is swallowed by accident, consult your doctor or pharmacist immediately or call the Toxicology Information Service, telephone 91 562 04 20, indicating the medicine and the amount used.
If you forget to use Menopur,do not use a double dose to make up for forgotten doses. Please inform your doctor or pharmacist.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects
Ovarian Hyperstimulation Syndrome (OHSS)
Tell your doctor immediatelyif you experience any of the following, which can be signs of OHSS:
- you have stomach pain or swelling
- you feel or are sick
- you have diarrhea
- You have gained weight
- you have difficulty breathing
- you need to urinate less often.
Tell your doctor immediately, even if it has been a few days since your last injection, or you stop using Menopur. You may need urgent medical treatment. These side effects can mean that your ovaries have been over-stimulated, which is known as ovarian hyperstimulation syndrome (OHSS). In severe cases of OHSS, fluid accumulation in the abdomen, pelvis or chest cavity, difficulty breathing, decreased urination, blood clots in the blood vessels (thromboembolism), and twisting of the ovaries (ovarian torsion) have been reported rarely.
Allergic reactions.
Tell your doctor immediately if you have:
- rash,
- itching
- swelling of the throat and difficulty breathing .
If you experience any of these symptoms, contact your doctor immediately.
Other side effects
Common side effects(may affect up to 1 in 10 people):
- headache
- feeling sick (nausea)
- stomach pain and swelling
- pelvic pain
- pain, redness, swelling, itching or bruising at the injection site.
Uncommon side effects(may affect up to 1 in 100 people):
- being sick (vomiting)
- stomach pain
- diarrhea
- feeling tired (fatigue)
- feeling dizzy
- fluid-filled sacs in the ovaries (ovarian cyst)
- breast complications (including breast pain, breast tenderness, breast discomfort, nipple pain and breast swelling)
- hot flushes
Rare side effects(may affect up to 1 in 1,000 people):
- spots (acne)
Other side effects(frequency not known):
- vision changes
- fever
- feeling unwell in general
- weight gain
- muscle and joint pain
- twisting of the ovaries as a complication of increased ovarian activity due to hyperstimulation.
- hives
- blood clots as a complication of increased ovarian activity due to hyperstimulation.
Reporting of side effects.
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Monitoring System for Human Use https://www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Menopur
Keep out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the pre-filled pen and on the carton after "EXP". The expiry date is the last day of the month indicated.
Before use:
Store in a refrigerator (2°C - 8°C).
Do not freeze.
After opening:
Use each pre-filled pen within 28 days of opening. Store below 25°C.
Always keep the pen with the cap on to protect it from light.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Container Contents and Additional Information
Composition ofMenopur
The active ingredient is highly purified menotropin (human menopausal gonadotropin, hMG)
A multidose pre-filled pen administers Menotropin corresponding to 1200 IU of follicle-stimulating hormone FSH activity and 1200 IU of luteinizing hormone LH activity.
Menotropin
The other components are:
- Phenol
- Methionine
- Arginine hydrochloride
- Polysorbate 20
- Sodium hydroxide
- Hydrochloric acid
- Water for injectable preparations
Appearance of the Product and Container Contents.
Menopur is a clear and colorless injectable solution in a pre-filled pen.
Menopur 1200 IU injectable solution in a pre-filled pen is available in packs of 1 pre-filled pen and 21 injection needles.
Marketing Authorization Holder and Manufacturer:
Marketing Authorization Holder
Ferring, S.A.U
C/ del Arquitecto Sánchez Arcas nº 3, 1º
28040 Madrid
Spain
Manufacturer: FERRING GmbH
Wittland 11,
D-24109 Kiel
Germany
This Medicinal Product is Authorized in the Member States of theEconomic European Areawith the Following Names:
Belgium, Ireland, Luxembourg, Slovakia: Menotrophin Ferring
Czech Republic: Menotrophin Ferring-Léciva
Bulgaria, Croatia, Cyprus, Denmark, Estonia, Finland, Greece, Hungary, Iceland, Latvia, Lithuania, Malta, Norway, Poland, Portugal, Romania, Slovenia, Spain, Sweden: Menopur
Germany: Menogon HP
Italy: Meropur
Date of the Last Revision of this LeafletJune 2022.
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
Instructions for Use
MENOPUR® Pre-filled Pen
Menopur injectable solution

Your healthcare professional (doctor, nurse, or pharmacist) should teach you how to prepare and inject Menopur properly before injecting it for the first time.
Read this manual completely before using the Menopur pre-filled pen and each time you acquire a new pen. There may be new information. Follow these instructions carefully, even if you have used a similar injectable pen before. Incorrect use of the pen may result in an incorrect dose of the medication.
Contact your healthcare professional if you have any questions about how to administer your Menopur injection.
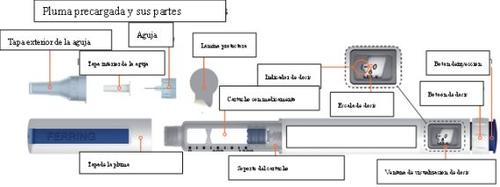
The Menopur pre-filled pen is a disposable, dosing pen that can be used for the administration of more than 1 dose of Menopur. The numbers you see in the dose display window represent the number of International Units (IU) of Menopur. The pen is available in 2 different concentrations:
- 600 IU • 1200 IU
Instructions for Use –Menopur Pre-filled Pen
- Important Information
- The Menopur pre-filled pen and needles are for use by one person only and should not be shared with others.
- Use the pen only for the medical condition for which it was prescribed and as your healthcare professional has instructed
- If you are blind or have poor vision, do not use this pen without help. Ask for help from a person with good vision and who is trained in how to use the pen.
Information about your Menopur pre-filled pen
The pen can be set to administer doses of 6.25 IU to 450 IU of Menopur in marked increments of 6.25 IU.
- The dose scale on the pen is numbered from 0 to 450 IU.
- Moving from one line that is labeled with a dose to the next line that does not have a dose label will increase or decrease the dose by 6.25 IU, depending on whether you are increasing or decreasing the dose. See “Examples of how to dial a dose” on pages 20 to 21.
- When turning the dose button to your dose, you will hear a click and feel resistance on the button for each increment to help you dial the correct dose.
Cleaning
- The outside of your pen may be cleaned with a damp cloth, if necessary.
- Do not put the pen in water or any other liquid.
Storage
- Do not freeze.
- Before using, store the pen in the refrigerator between 2 °C and 8 °C.
- After the first use, use each pre-filled pen within 28 days and store it below 25 °C.
- Always store the pen with the cap on and without a needle.
- Do not use the pen after the expiration date (EXP) printed on the pen label. The expiration date is the last day of the expiration month.
- Do not store the pen at extreme temperatures, direct sunlight, or very cold conditions, such as in a car or freezer.
- Store the pen out of sight and reach of children.
Items You Will Need to Administer Your Menopur Injection
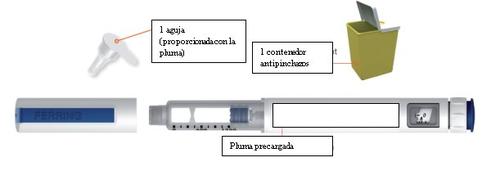
Before Use – (Step 1)
1.
- Wash your hands.
- Make sure you have the correct pen with the correct concentration.
- Check the expiration date on the pen label.
Attaching the Needle – (Steps 2 to 6)
Important:
- Always use a new needle for each injection.
- Use only the click-on needles for single use that are provided with the pen.
2.
- Remove the pen cap.
- Check the pen to see that it is not damaged.
- Check that the medication is clear and does not contain particles.
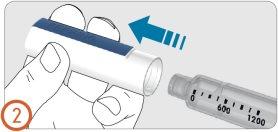 Do not use a pen if it is damaged, contains particles in the medication cartridge, or is cloudy.
Do not use a pen if it is damaged, contains particles in the medication cartridge, or is cloudy.
3.
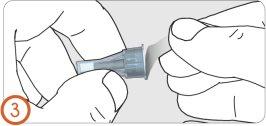 Remove the needle protective cap.
Remove the needle protective cap.
4.
- Attach the needle to the pen.
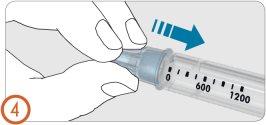 You will hear or feel a “click” when the needle is securely attached.
You will hear or feel a “click” when the needle is securely attached.
5.
- Remove the outer needle protective cap.
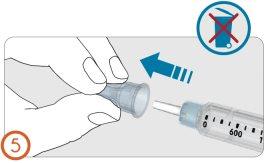 Do not discard the outer needle cap. You will need it to discard the needle after injecting the medication.
Do not discard the outer needle cap. You will need it to discard the needle after injecting the medication.
6.
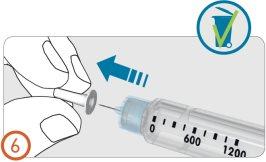 Remove the inner needle cap and discard it.
Remove the inner needle cap and discard it.
- Preparation – (Steps 7 to 9)
- Before using the pen for the first time, you will need to prime the cartridge (prepare) to receive the correct dose of medication.
- You should only prepare the pen the first time you use it.
- Perform steps 7 to 9 even if you do not see air bubbles.
- If the pen has already been used, go directly to step 10.
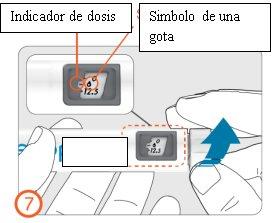
7.
- Turn the dose button clockwise until the drop symbol is aligned with the dose indicator.
- If you dial the wrong preparation dose, it can be corrected both up and down without loss of medication, by turning the dose button in any direction until the drop symbol is aligned with the dose indicator.
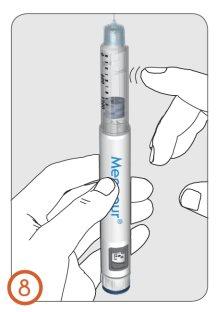
8.
- Hold the pen with the needle pointing upwards.
- Tap the cartridge support with your finger to make any air bubbles present in the cartridge rise to the top of the cartridge.
9.
- With the needle still pointing upwards (away from your face), press the injection button until you see the number ‘0’ in line with the dose indicator.
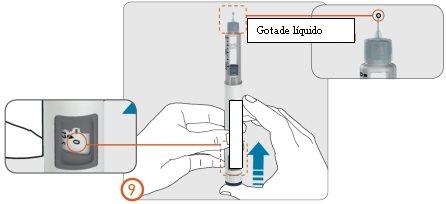
- Check that a drop of liquid appears at the tip of the needle.
- If the drop(s) do not appear, repeat steps 7 to 9 (Preparation) until a drop appears.
- If a drop does not appear after 5 attempts, remove the needle (see step 13), and attach a new needle (see steps 3 to 6), and repeat the preparation (see steps 7 to 9).
- If you still do not see a drop after using a new needle, try with a new pen.
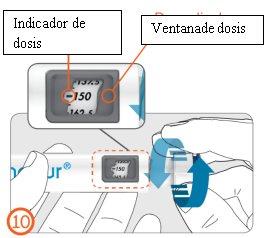
Dialing the Dose – (Step 10)
- Turn the dose button clockwise until the prescribed dose is aligned with the dose indicator in the dose display window. The dose can be corrected both up and down without loss of medication, by turning the dose button in any direction until the correct dose is aligned with the dose indicator. Do not press the injection button when dialing a dose to avoid loss of medication.
See “Examples of how to dial a dose” on pages 20 to 21.
Dose Division
- You may need more than one pen to complete the dose that has been prescribed for you.
- If you are unable to dial your complete dose, this means that there is not enough medication left in the pen. You will need to administer the dose by dividing it into two injections or discard your pen and use a new one for your injection.
See “Giving a divided dose of Menopur“ on pages 22 to 23 for examples of how to calculate and record your divided dose.
.
- Injecting the Dose – (Steps 11 to 12)
- Important:
- Read steps 11 and 12 on pages 14 to 15 before administering your injection.
- This medication should be administered by injection just under the skin (subcutaneously) in the stomach area (abdomen).
- Use a new injection site for each injection to minimize the risk of skin reactions such as redness and irritation.
- Do not inject into an area that is irritated (sensitive), bruised, red, hard, scarred, or has stretch marks.
11.
- Hold the pen so that the dose display window is visible during the injection.
- Pinch your skin and insert the needle directly into the skin as your healthcare professional has taught you. Do not press the injection button yet. (see step 11)
- After inserting the needle, place your thumb on the injection button.
 Press the injection button all the way down and hold it.
Press the injection button all the way down and hold it.
- Continue to hold the injection button down and when you see the number ‘0’ in line with the dose indicator, wait 8 seconds (counting slowly to 8) (see step 12). This will ensure that you receive your complete dose.
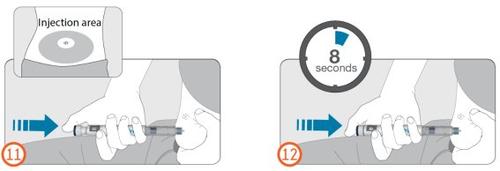
12.
- After pressing the injection button for 5 seconds, release it. Then, slowly remove the needle from the injection site, pulling it straight out of the skin.
- If blood appears at the injection site, press the area lightly with a gauze or a cotton ball.
Note:
- Do not tilt the pen during the injection or during the removal of the needle from the skin.
- Tilting the pen can cause the needle to bend or break.
- If a broken needle remains stuck in the body or under the skin, seek immediate medical help.
Disposing of the Needle – (Step 13)
13.
- Carefully replace the outer needle cap with a firm push (see step 13A).
- Unscrew the needle in a counterclockwise direction to remove it from the pen (see steps 13B and 13C).
- Carefully dispose of the used needle (see step 13AD).
- See “Disposal” on page 18.
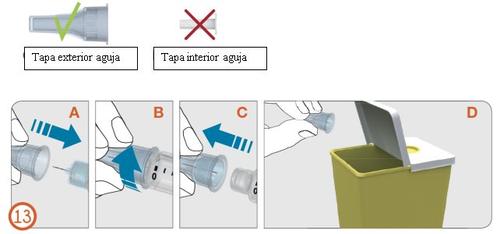
Note:
- Always remove the needle after each use. Needles are for single use only.
- Do not store the pen with the needle attached.
.
Replacing the Cap on the Pen – (Step 14)
14.
- Replace the pen cap firmly to protect it between injections
Note:
- The pen cap will not fit if a needle is attached.
 Keep the pen cap on when not in use.
Keep the pen cap on when not in use.
Disposal
Needles:Place used needles in a puncture-resistant container, such as a sharps container, immediately after use.
If you do not have a sharps container, you may use a household container that has the following characteristics:
- It is made of heavy-duty plastic,
- it can be closed with a tight-fitting, puncture-resistant lid, without the puncture being able to get out,
- it can stand upright and stable during use,
- it is leak-proof, and
- it is properly labeled to warn that it contains hazardous waste.
You should dispose of the sharps container when it is almost full. Ask your doctor, nurse, or pharmacist what the right way to dispose of it is. Do not dispose of your used sharps container in household trash unless your community guidelines permit it.
Menopur Pre-filled Pens:
- Ask your pharmacist how to dispose of unused medications.
In the Following Pages, You Will Find More Information on the Topics Listed Below.1:• Examples of how to dial a dose...............................................................page 20 to 21 • Administration of a divided dose of Menopur.....................page 22 • Divided dose diary......................................................................... page 23 • Frequently Asked Questions (FAQ)............................................................page 24 • Warnings..................................................................................................page 25
- Contact...........................................................................................................page 25
Examples of How to Dial a Dose
Examples of how to dial a dose using your Menopur pre-filled pen
The graph on the right shows examples of prescribed doses, how to dial the examples of prescribed doses, and how the dose display window looks for the prescribed doses.
Examples of prescribed doses (in IU) (units) | Priming dose | 75 |
Dose to dial on pen | Priming symbol ((Dial a click starting from 0 until you see the priming drop symbol)) | 75 (Dial to 75) |
Dose display window for example prescribed dose |
|
|
118.75 | 225 | 262.5 | 343.75 | 450 |
118.75 (Dial to 112.5 plus 1 click) | 225 (Dial to 225) | 262.5 (Dial to 262.5) | 343.75 (Dial to 337.5 plus 1 click) | 450 (Dial to 450) |
|
|
|
|
|
Administration of a split dose ofMenopur
If you are unable to dial the complete dose that has been prescribed for you on your pen, this means that there is not enough medication left in the pen to administer the complete dose. You will need to administer part of your prescribed dose using the pen you were using, and the rest of the dose using a new pen (split dose injection) or you will need to discard the pen you were using and use a new pen to administer the complete dose that has been prescribed for you in a single injection. If you decide to administer the split dose in two injections, follow these instructions, and write down the amount of medication to be administered using the split dose diary on page 231.
- Column A shows an example of a prescribed dose. Write down your prescribed dose in Column A.
- Column B shows an example of the dose remaining in the pen (this is the same as what you can dial).
- Write down the dose remaining in your pen in Column B. Administer the injection using the remaining amount of medication in your pen.
- Prepare and prime a new pen (Steps 1 to 9).
- Calculate and write down the remaining dose to inject in Column C by subtracting the number in Column B from the number in Column A. Use a calculator to verify your calculations if necessary.
- Refer to “Examples of how to dial a dose” on pages 20 to 211 if necessary.
- Contact your healthcare professional if you have any doubts about how to calculate your split dose.
- • Inject the remaining dose of medication (the number in Column C) using your new pen to complete the prescribed dose.
Split Dose Diary
A Prescribed dose | B Dose remaining in the pen (Dose shown on the dose indicator in the dose display window) | C = A minus B Dose to inject in new pen (Dose shown on the dose indicator in the dose display window) |
112.5 | 75 (75) | 37.5 (37.5) |
125 | 50 (50) | 75 (75) |
300 | 181.25 (175 plus 1 line) | 118.75 (112.5 plus 1 line) |
Frequently Asked Questions (Q&A)
- Is the preparation step necessary before each injection?
- No. Preparation should only be done before the administration of the first injection with a new pen.
- How can I tell if the injection is complete?
- The injection button has been pressed firmly to the end until it stops.
- The number ‘0’ is in line with the dose indicator.
- You have counted slowly to 8 while holding the injection button and the needle is still injected in the skin.
.
- Why should I count to 8 while holding the injection button?
- Holding the injection button for 8 seconds allows the complete dose to be injected and absorbed under your skin.
.
- What happens if the dose button cannot be turned to the prescribed dose?
- It is possible that the pen cartridge does not have enough medication to deliver the prescribed dose.
- The pen will not allow you to dial a dose greater than what is left in the cartridge.
- You can inject the amount of medication left in the pen and complete the prescribed dose with a new pen (split dose) or use a new pen to administer the complete prescribed dose.
.
- What if I don't have enough needles?
- • If you need additional needles, contact your healthcare professional. Use only the needles that come with the pre-filled Menopur pen or those prescribed by your healthcare professional.
Precautions
- Do not use a pen that has been dropped or hit against hard surfaces.
- If it is not easy to press the injection button, do not force it. Change the needle. If the injection button is still not easy to press after changing the needle, use a new pen.
- Do not attempt to repair a damaged pen. If a pen is damaged, contact your healthcare professional or local representative of the marketing authorization holder.
Contact
If you have any questions or problems related to the pen, contact your healthcare professional or local representative of the marketing authorization holder.
Marketted by: Ferring SAU
C/ Arquitecto Sánchez Arcas nº3, 1º
28040 Madrid
Spain
Revised: June 2022
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MENOPUR 1200 IU PRE-FILLED PEN SOLUTION FOR INJECTIONDosage form: INJECTABLE, -Active substance: human menopausal gonadotrophinManufacturer: Angelini Pharma Espana S.L.Prescription requiredDosage form: INJECTABLE, 1200 IUActive substance: human menopausal gonadotrophinManufacturer: Ferring S.A.Prescription requiredDosage form: INJECTABLE, 600 IUActive substance: human menopausal gonadotrophinManufacturer: Ferring S.A.Prescription required
Online doctors for MENOPUR 1200 IU PRE-FILLED PEN SOLUTION FOR INJECTION
Discuss questions about MENOPUR 1200 IU PRE-FILLED PEN SOLUTION FOR INJECTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions













