How to use Menopur
PATIENT INFORMATION LEAFLET
For Internal Use - Internal
Enclosed patient information leaflet
MENOPUR 600 IU solution for injection in a pre-filled pen injector
Menotropin
Read the patient information leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for your use only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What MENOPUR is and what it is used for
- 2. Important information before using MENOPUR
- 3. How to use MENOPUR
- 4. Possible side effects
- 5. How to store MENOPUR
- 6. Contents of the pack and other information
1. What MENOPUR is and what it is used for
MENOPUR is a solution for injection in a pre-filled pen injector. The injection is given under the skin (subcutaneously) - usually in the abdomen. MENOPUR contains menotropin, which is a mixture of two natural hormones called follicle-stimulating hormone (FSH) and luteinizing hormone (LH). They enable the normal functioning of the reproductive organs. The FSH and LH hormones contained in menotropin are obtained from the urine of postmenopausal women.
- hormone follicle-stimulating (FSH) and
- hormone luteinizing (LH).
What MENOPUR is used for
MENOPUR is used to treat women who cannot become pregnant, in the following situations: in women who cannot become pregnant because their ovaries do not produce eggs (also in the case of polycystic ovary syndrome). MENOPUR is used in women who have been treated for infertility with a medicine called "clomiphene citrate" but it has been ineffective; in women participating in assisted reproduction programs, such as in vitro fertilization (IVF) or embryo transfer (ET), gamete intrafallopian transfer (GIFT), or intracytoplasmic sperm injection (ICSI).
How MENOPUR works
MENOPUR stimulates the ovaries to produce multiple follicles, in which eggs can develop. This is called the development of multiple follicles. For Internal Use - Internal
2. Important information before using MENOPUR
What to check before starting MENOPUR treatment
Before starting MENOPUR treatment, your doctor must assess the causes of fertility disorders in both partners. In particular, check if the following diseases do not occur, which require different, appropriate treatment: hypothyroidism and adrenal insufficiency; high levels of a hormone called prolactin - hyperprolactinemia; pituitary tumors - a gland located at the base of the brain; hypothalamic tumors - an area located under the part of the brain called the hill. If any of the above diseases are found in the patient, the doctor must be informed about this before starting MENOPUR treatment.
When not to use MENOPUR
if the patient is allergic to menotropin or any of the other ingredients of this medicine (listed in section 6); if the patient has tumors of the uterus, ovaries, breast, or parts of the brain, such as the pituitary gland or hypothalamus; if the patient has ovarian cysts or ovarian enlargement - not caused by polycystic ovary syndrome; if the patient has developmental abnormalities of the uterus or other genital organs; if the patient has vaginal bleeding of unknown cause; if the patient has uterine fibromyomas - these are non-cancerous tumors in the uterus; if the patient is pregnant or breastfeeding.
Warnings and precautions Ovarian Hyperstimulation Syndrome (OHSS)
A serious side effect of this medicine, especially in women with polycystic ovary syndrome, is "ovarian hyperstimulation syndrome" or "OHSS" (see section 4). You should immediately inform your doctor if you experience OHSS symptoms, even if: it has been several days since the last dose of the medicine; you have stopped using MENOPUR. These may be symptoms of excessive ovarian activity, which can be severe. In such a case, the doctor will discontinue MENOPUR treatment and initiate appropriate hospital treatment.
- if it has been several days since the last dose of the medicine
- if you have stopped using MENOPUR.
Taking the recommended dose and carefully monitoring the treatment reduces the likelihood of these symptoms.
Tests and examinations
During MENOPUR treatment, your doctor will usually refer you for ultrasound examinations (USG) and sometimes blood tests to check your response to treatment.
Pregnancy-related risks
Hormone treatment, such as MENOPUR, may increase the risk of: ectopic pregnancy in women with previously diagnosed fallopian tube diseases; miscarriage; multiple pregnancy (twins, triplets, etc.); physical defects present in the child at birth (congenital defects). In some women treated for infertility, ovarian or other reproductive organ tumors have developed. It is not yet known whether this was caused by hormone treatment such as MENOPUR. For Internal Use - Internal
Blood clots
The likelihood of blood clots in veins or arteries is higher in pregnant women. Infertility treatment may increase the likelihood of blood clots, especially if: you are overweight; you have a blood clotting disorder "thrombophilia"; blood clots have occurred before or in someone in your family. You should inform your doctor if you think this applies to you.
Children and adolescents
Using the MENOPUR medicinal product in children and adolescents is not appropriate.
MENOPUR and other medicines
Tell your doctor about all medicines you are taking now or have recently taken, as well as any medicines you plan to take. Clomiphene citrate is another medicine used to treat infertility. If MENOPUR is given at the same time as clomiphene citrate, the effect on the ovaries may be enhanced.
Pregnancy and breastfeeding
MENOPUR should not be used during pregnancy or breastfeeding.
Driving and using machines
It is unlikely that MENOPUR will affect your ability to drive or use machines.
Important information about some ingredients of MENOPUR
MENOPUR contains less than 1 mmol of sodium (23 mg) per dose, which means that the medicine is essentially "sodium-free".
3. How to use MENOPUR
This medicine should always be used exactly as your doctor has told you. If you are not sure, ask your doctor.
Women who do not produce eggs (do not ovulate)
Treatment should start within the first 7 days of the menstrual cycle. Day 1 is the first day of menstruation. The medicine should be given daily for at least 7 days. How much MENOPUR to use? The initial dose is usually 75 IU (International Units) to 150 IU per day. Depending on the patient's response, the dose can be increased - up to a maximum of 225 IU. The prescribed dose should be given for at least 7 days before the doctor changes the dose. It is recommended to increase the dose by 37.5 IU, but not more than 75 IU, each time. The doctor will check the treatment results with MENOPUR. The treatment cycle should be discontinued if no response to treatment is found after 4 weeks. When the response to MENOPUR treatment is satisfactory: the patient will receive a single injection of a hormone called "human chorionic gonadotropin" (hCG). The dose will be 5,000 IU to 10,000 IU. The patient will receive hCG the day after the last MENOPUR injection. For Internal Use - Internal It is recommended to have sexual intercourse on the day of injection and the next day after hCG injection. Alternatively, insemination (insemination directly into the uterus) can be performed. The patient remains under the doctor's close observation for at least 2 weeks. When there is no response to MENOPUR treatment: the doctor will check the treatment results. Depending on the progress of the treatment, the doctor may decide to discontinue MENOPUR administration and refrain from administering hCG.
Women participating in assisted reproduction programs
Patients participating in an assisted reproduction program will also receive a medicine that supports the action of a hormone called "gonadotropin-releasing hormone", GnRH. This medicine is called a "GnRH agonist". MENOPUR administration should start about 2 weeks after starting GnRH agonist treatment. The patient may also take a medicine called a "GnRH antagonist". MENOPUR administration should start on the 2nd or 3rd day of the menstrual cycle (1st day of menstrual bleeding is the 1st day of the cycle). How much MENOPUR to use? MENOPUR should be given daily for at least 5 days. The initial dose of MENOPUR is usually 150 IU to 225 IU. Depending on the patient's response to treatment, this dose may be increased to a maximum of 450 IU per day. The dose should not be increased by more than 150 IU at a time. Treatment usually should not last longer than 20 days. After finding a sufficient number of appropriately sized follicles, the patient will receive a single injection of hCG at a dose of up to 10,000 IU to induce ovulation (egg release). The patient remains under the doctor's close observation for at least 2 weeks after hCG administration. The doctor will check the treatment results with MENOPUR. Depending on the progress of the treatment, the doctor may decide to discontinue MENOPUR administration and refrain from administering hCG. In such a case, the patient will be instructed to use a mechanical contraceptive method (e.g., a condom). Otherwise, you should refrain from sexual intercourse until the next menstruation.
Using MENOPUR
Follow the "Instructions for use" provided with the pen injector package carefully. The first MENOPUR injection should be performed under the supervision of a doctor or nurse. The doctor will decide whether the patient can perform subsequent injections at home - after complete training. MENOPUR will be given as a subcutaneous injection, usually in the abdomen. Each pre-filled pen injector can be used for multiple injections.
Using a higher dose of MENOPUR than recommended
If a higher dose of MENOPUR than recommended is used, inform your doctor. For Internal Use - Internal
Missing a dose of MENOPUR
Do not take a double dose to make up for a missed dose. If you miss a dose of MENOPUR, inform your doctor.
4. Possible side effects
Like all medicines, MENOPUR can cause side effects, although not everybody gets them.
Serious side effects
Ovarian Hyperstimulation Syndrome (OHSS)
Immediately inform your doctorif you experience any of the following symptoms, which may be symptoms of OHSS: abdominal pain or swelling; nausea; diarrhea; weight gain; breathing difficulties; decreased urination frequency.
If you experience any of these symptoms, immediately contact your doctor, even if the symptom occurs several days after the last dose of the medicine or if you have stopped using MENOPUR.
The patient may need urgent medical attention. These side effects may indicate excessive ovarian activity, which is known as ovarian hyperstimulation syndrome (OHSS). In severe cases of OHSS, rare complications have occurred, such as fluid accumulation in the abdominal cavity, pelvic cavity, or pleural cavity, breathing difficulties, and decreased urination frequency or amount, blood clots in blood vessels (thromboembolic disorders), and ovarian torsion.
Allergic reactions (hypersensitivity)
Immediately inform your doctorif you experience: rash; itching; swelling of the throat and breathing difficulties. If you experience any of these symptoms, immediately contact your doctor.
Other side effects
The following side effectsoccur frequently, i.e., in 1 to 10 out of every 100 treated patients: headache; nausea; abdominal pain or swelling; pelvic pain; reactions at the injection site, such as pain, redness, swelling, itching, or bruising. The following side effectsoccur less frequently, i.e., in 1 to 10 out of every 1,000 treated patients: vomiting; abdominal discomfort; diarrhea; fatigue; dizziness; ovarian cysts; breast symptoms, including breast pain, tenderness, discomfort, nipple pain, and breast swelling; hot flashes. The following side effectsoccur rarely, i.e., in 1 to 10 out of every 10,000 treated patients: acne. In addition to those listed above, the following side effectshave been observed, with unknown frequency: vision disorders; fever; nausea; weight gain; muscle and joint pain; ovarian torsion, as a complication of increased ovarian activity caused by excessive stimulation; hives; blood clots, as a complication of increased ovarian activity caused by excessive stimulation.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor. Side effects can be reported directly to the Department of Drug Adverse Reaction Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Aleje Jerozolimskie 181C, 02-222 Warsaw, Tel: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store MENOPUR
Keep this medicine out of the sight and reach of children. Do not use this medicine after the expiry date which is stated on the label of the pre-filled pen injector and the carton after "EXP". The expiry date refers to the last day of that month. Before use: Store in a refrigerator (2°C - 8°C). Do not freeze. After opening: Use each pre-filled pen injector within 28 days of opening. Store below 25°C. Always store the pen injector with the cap on to protect it from light. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
For Internal Use - Internal
What MENOPUR contains
- The active substance is highly purified menotropin (human menopausal gonadotropin, hMG)
MENOPUR 600 IU solution for injection in a pre-filled pen injector: One pre-filled pen injector contains menotropin in an amount equivalent to 600 IU FSH (follicle-stimulating hormone) and 600 IU LH (luteinizing hormone). The other ingredients are: phenol; methionine; arginine hydrochloride; polysorbate 20; sodium hydroxide; hydrochloric acid; water for injections
What MENOPUR looks like and contents of the pack
MENOPUR is a clear and colorless solution for injection in a pre-filled pen injector. MENOPUR 600 IU solution for injection in a pre-filled pen injector is available in packs containing 1 pen injector and 12 injection needles.
Marketing authorization holder and importer:
Ferring GmbH, Wittland 11, D-24109 Kiel, Germany. Local representative of the marketing authorization holder: Ferring Pharmaceutical Poland Sp. z o.o., ul. Szamocka 8, 01-748 Warsaw, Tel.: +48 22 246 06 80
This medicine is authorized in the Member States of the European Economic Area under the following names:
Belgium, Czech Republic, Ireland, Luxembourg, Slovakia: Menotropin Ferring; Bulgaria, Croatia, Cyprus, Denmark, Estonia, Finland, Greece, Hungary, Iceland, Latvia, Lithuania, Malta, Norway, Poland, Portugal, Romania, Slovenia, Spain, Sweden: Menopur; Germany: Menogon HP; Italy: Meropur; Date of last revision of the leaflet:July 2024; For Internal Use - Internal; Instructions for use
MENOPUR pre-filled pen injector
Menotropin, solution for injection

Before the first injection, a medical professional (doctor, nurse, or pharmacist) should demonstrate how to properly prepare and inject MENOPUR. Read this instruction carefully before using the MENOPUR pen injector and every time you receive a new pen injector. They may contain new information. Follow this instruction completely, even if you have used a similar pen injector before. Improper use of the pen injector can result in an incorrect dose of the medicine.
Important information about the MENOPUR pen injector
The pen injector allows you to set doses from 6.25 IU to 450 IU of MENOPUR, in increments of 6.25 IU.
- The dose scale on the pen injector has a numerical scale from 0 to 450 IU.
- Moving from one marked dose increment to the next unmarked increment will increase or decrease the dose by 6.25 IU, depending on whether you are increasing or decreasing the dose. See "Examples illustrating how to set the dose" on pages 20-21.
- When turning the dose-setting knob, you will hear a click and feel resistance on the knob before each subsequent dose increase, which helps you set the correct dose.
Cleaning
- If necessary, the outer surface of the pen injector can be cleaned with a water-moistened cloth.
- Do not put the pen injector in water or any other liquid.
Storage
- Do not freeze.
- Before use, store the pen injector in a refrigerator at a temperature between 2°C and 8°C.
- After first use, the pen injector can be stored for a maximum of 28 days at a temperature below 25°C.
- Always store the pen injector with the cap on and without a needle attached.
- Do not use the pen injector after the expiry date (EXP) printed on the label. The expiry date refers to the last day of that month.
- Do not store the pen injector in extreme temperatures or places exposed to direct sunlight or very low temperatures, such as in a car or freezer. Store the pen injector out of the reach of children.
Materials needed for injection
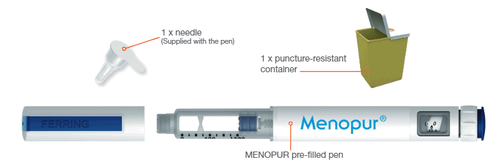
For Internal Use - Internal
Before use - (Step 1)
1.
- Wash your hands.
- Make sure you are using a pen injector with the correct strength.
- Check the expiry date on the pen injector label.
Attaching the needle - (Steps 2-6)
Important:
- Always attach a new needle for each injection.
- Only use single-use needles with a locking mechanism, which come with the pen injector.
For Internal Use - Internal
2.
- Remove the pen injector cap.
- Check if the pen injector is damaged.
- Check if the solution is clear and does not contain undissolved particles.
- Do not use the pen injector if it is damaged, contains undissolved particles, or the solution is cloudy.
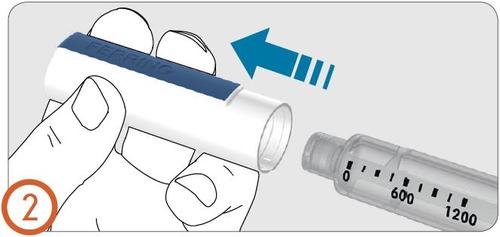
3.
- Remove the needle protective cap.
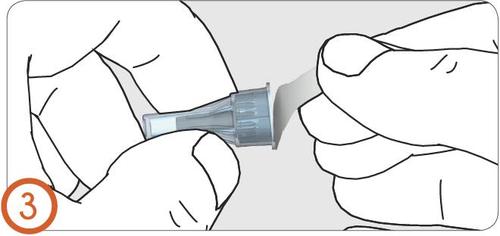
4.
For Internal Use - Internal
- Insert the needle into the pen injector.
- When the needle is securely attached, you should hear or feel a click.
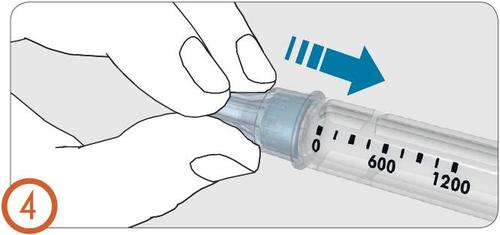
5.
- Remove the outer needle cap.
- Do notdiscard the outer needle cap. It will be needed to remove and dispose of the needle after injection.
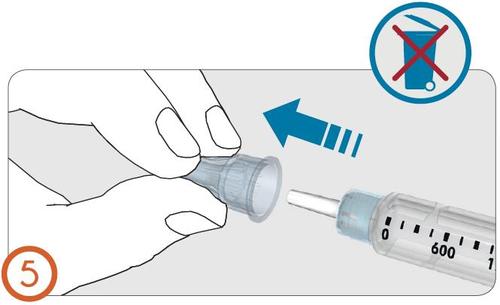
6.
- Remove and discard the inner needle cap.
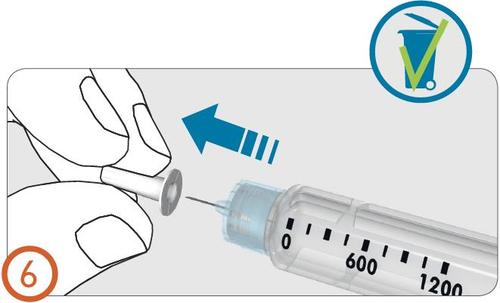
Removing air bubbles - (Steps 7-9)
- Before using the pen injector for the first time, you need to remove air bubbles from the cartridge to ensure proper dose setting.
- Air bubbles should only be removed before the first use of the pen injector.
- You must perform Steps 7-9, even if you do not see air bubbles.
- If the pen injector has been used before, proceed directly to Step 10.
7.
- Turn the dose-setting knob clockwise until the drop symbol is in line with the dose indicator.
- If an incorrect dose is set for air bubble removal, the dose can be corrected up or down without losing medicine by turning the dose-setting knob in either direction until the drop symbol is in line with the dose indicator.
For Internal Use - Internal
Dose indicator
Drop symbol
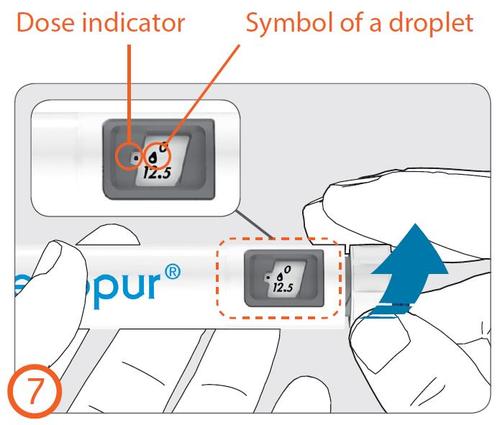
Dose indicator
Drop symbol
8.
- Hold the pen injector upright with the needle facing upwards.
- Tap the cartridge with your finger to move any air bubbles in the cartridge to the top.
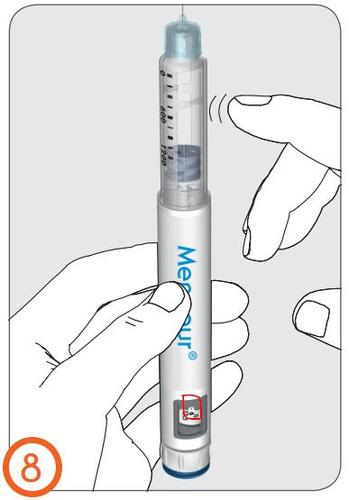
9.
- Holding the pen injector with the needle still facing upwards (away from your face), press the dose button all the way until the number "0" is in line with the dose indicator. Check if a drop of liquid appears at the tip of the needle.
- If a drop does not appear, repeat Steps 7-9 (air bubble removal) until a drop appears.
- If a drop still does not appear after 5 attempts, remove the needle (see Step 13), attach a new needle (see Steps 3-6), and repeat the air bubble removal (see Steps 7-9).
- If a drop still does not appear after using a new needle, try a new pen injector.
Setting the dose - (Step 10)
- Turn the dose-setting knob clockwise until the prescribed dose is in line with the dose indicator in the dose window.
- The dose can be adjusted up or down without losing medicine by turning the dose-setting knob in either direction until the correct dose is in line with the dose indicator.
- Do not press the dose button while setting the dose to avoid losing medicine. See "Examples illustrating how to set the dose" on pages 20-21.
Dose indicator
Window displaying the dose
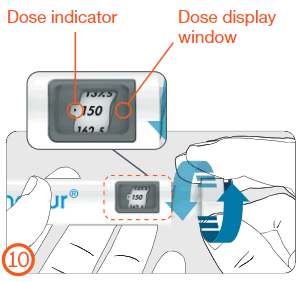
For Internal Use - Internal
where examples are given on how to calculate and record a divided dose.
Injecting the dose - (Steps 11-12)
Important:
- Read the description of Steps 11 and 12 on pages 14-15 before performing the injection.
- The medicine should be injected under the skin (subcutaneously) in the abdominal area near the navel.
- Each subsequent injection should be performed in a different location to reduce the risk of skin reactions, such as redness and irritation.
- Do not inject into an area that is painful, bruised, red, hard, scarred, or has stretch marks.
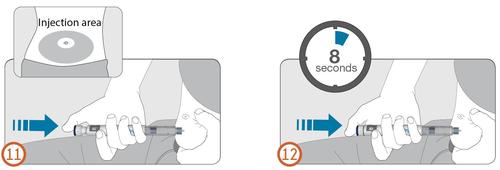
11.
- Hold the pen injector so that the dose window is visible during injection.
- Grasp a skin fold and insert the needle straight into the skin, as demonstrated by a medical professional. Do not press the dose button yet. (See Figure 11).
- Place your thumb on the dose button.
- Press the dose button all the way and hold it.
- Hold the dose button pressed and when the number "0" is in line with the dose indicator, wait for 8 seconds (count slowly to eight). (See Figure 12.) This ensures that the full dose is administered.
seconds
12.
- After holding the dose button pressed for 8 seconds, release the dose button. Then, slowly remove the needle from the injection site, pulling it straight out of the skin.
- If blood appears at the injection site, press a swab or cotton ball gently against the injection site.
Warning:
- Do not tilt the pen injector during injection and needle removal.
- Tilting the pen injector can cause the needle to bend or break.
- If the broken needle remains in the body or under the skin, seek medical attention immediately.
Removing the needle - (Step 13)
13.
For Internal Use - Internal
- Carefully put the outer needle cap back on the needle, pushing it firmly (see Figure 13A).
- Unscrew the needle by turning it counterclockwise to detach it from the pen injector (see Figures 13B and 13C).
- Dispose of the used needle carefully (see Figure 13D).
- See "Disposal" on page 18.
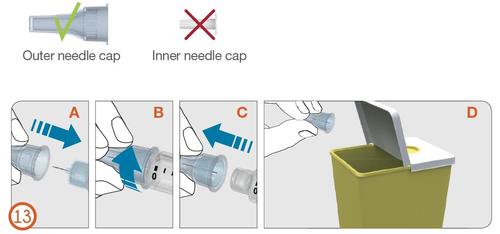
Outer needle cap
Inner needle cap
Warning:
- Always remove the needle after each use. Needles are for single use only.
- Do not store the pen injector with a needle attached.
Putting the pen injector cap back on - (Step 14)
14.
- Put the pen injector cap back on the pen injector firmly to protect it between injections.
Warning:
- The pen injector cap will not fit on the needle.
- When the pen injector is not in use, it should be protected with a cap.
Disposal
Needles:
Used needles should be placed in a puncture-resistant container, such as a sharps container, immediately after use. For Internal Use - Internal
If a sharps container is not available, a container that meets the following requirements can be used:
- it is made of sturdy plastic,
- it can be sealed with a puncture-resistant lid to prevent sharps from escaping,
- it stands upright and remains stable during use,
- it is leak-proof, and
- it is properly labeled as containing hazardous waste inside the container.
The sharps container should be disposed of when it is almost full. Ask your doctor, nurse, or pharmacist about the proper disposal method. Do not dispose of a filled sharps container in the household trash, unless local regulations permit it.
MENOPUR pen injectors:
- Ask your pharmacist how to dispose of medicines that are no longer needed.
The following information can be found on the following pages:
:
- Examples illustrating how to set the dose.................. pages 20-21
- Administering a divided dose of MENOPUR.................. page 22
- Divided doses .................................................. page 23
And also:
- Frequently asked questions……………........................... page 24
- Warnings............................................................................ page 25
- Contact.................................................................................. page 25
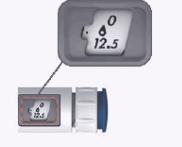
Examples illustrating how to set the dose
Examples illustrating how to set the dose on the MENOPUR pen injector.
The table on the right shows examples of prescribed doses, how to set the example prescribed doses, and what the dose window looks like for the example prescribed doses.

For Internal Use - Internal





Administering a divided dose of MENOPUR
If it is not possible to set the full prescribed dose on the pen injector, it means that there is not enough medicine left in the pen injector to administer the full dose. You will need to administer part of the prescribed dose using the current pen injector and the remaining part of the dose using a new pen injector (divided dose injection). Alternatively, you can dispose of the current pen injector and use a new pen injector to administer the full prescribed dose in one injection. If you choose to administer a divided dose, follow the instructions below and record the amounts of medicine to be administered as shown in the divided dose log on page 23.
- Column A shows examples of prescribed doses. Record the prescribed dose in Column A.
- Column B shows examples of the dose value remaining in the pen injector (which is the value that can be set on the pen injector).
- Record the dose value remaining in the pen injector in Column B. Perform the injection using the remaining medicine in the pen injector.
- Prepare and prime a new pen injector (Steps 1-9).
- Calculate and record the remaining dose to be injected in Column C, subtracting the value from Column B from the value from Column A. If necessary, check your calculations on a calculator.
- If necessary, see "Examples illustrating how to set the dose" on pages 20-21.
- If you are unsure about calculating the divided dose, contact a medical professional.
- Inject the remaining part of the dose (the value from Column C) using the new pen injector to complete the prescribed dose.
Divided dose log
Most Frequently Asked Questions
For Internal Use - Internal
- 1. Do I need to vent the injector before each injection?
- No. Venting must be performed only before administering the first injection with a new injector.
- 2. How will I know that the injection is complete?
- The dose button is pressed all the way down.
- The number "0" is set on the dose indicator line.
- Count slowly to 8 while holding the dose button down with the needle still inserted under the skin.
- 3. Why do I need to count to 8 while holding the dose button down?
- Holding the dose button down for 8 seconds allows for the injection of the entire dose and its absorption under the skin.
- 4. What if the dose setting dial cannot be set to the required dose?
- The cartridge in the injector may not contain enough medication to administer the prescribed dose.
- The injector does not allow setting a dose larger than the dose remaining in the cartridge.
- You can inject the remaining medication in the injector and complete the administration of the prescribed dose using a new injector (split dose) or use a new injector to administer the entire prescribed dose.
- 5. What if I don't have enough needles?
- If you need additional needles, you should contact your doctor. You should only use the needles provided with the MENOPUR injector or prescribed by your doctor.
Warnings
- Do not use an injector that has been dropped or has hit a hard surface.
- If the dose button does not press down easily, do not force it. You should change the needle. If the dose button still does not press down easily after changing the needle, you should use a new injector.
- Do not attempt to repair a damaged injector. If the injector is damaged, you should contact a medical professional or your local representative of the responsible entity.
Contact
In case of any questions or problems with the injector, you should contact a medical professional or the representative of the responsible entity.
The local representative of the responsible entity:
Ferring Pharmaceutical Poland Sp. z o.o.
Szamocka 8
01-748 Warsaw
Phone: + 48 22 246 06 80
Last update: March 2025
For Internal Use - Internal
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterFerring GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MenopurDosage form: Powder, 75 IU FSH + 75 IU LHActive substance: human menopausal gonadotrophinManufacturer: Ferring GmbHPrescription requiredDosage form: Powder, 600 IU FSH + 600 IU LHActive substance: human menopausal gonadotrophinPrescription requiredDosage form: Powder, 1200 IU FSH + 1200 IU LHActive substance: human menopausal gonadotrophinPrescription required
Alternatives to Menopur in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Menopur in Іспанія
Alternative to Menopur in Україна
Online doctors for Menopur
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Menopur – subject to medical assessment and local rules.