
Cixodil
Ask a doctor about a prescription for Cixodil

How to use Cixodil
Leaflet accompanying the packaging: information for the user
Cyxodil, 80 micrograms/dose, inhalation aerosol, solution
Cyxodil, 160 micrograms/dose, inhalation aerosol, solution
Cyxodil, 320 micrograms/dose, inhalation aerosol, solution
Ciclesonide
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet:
- 1. What is Cyxodil and what is it used for
- 2. Important information before using Cyxodil
- 3. How to use Cyxodil
- 4. Possible side effects
- 5. How to store Cyxodil
- 6. Contents of the packaging and other information
1. What is Cyxodil and what is it used for
What is Cyxodil:
Cyxodil contains ciclesonide as the active substance. Cyxodil is a clear and colorless inhalation aerosol intended for inhalation through the mouth into the lungs. It is a preventive medicine (corticosteroid) that should be used daily and its effect occurs only after inhalation into the lungs.
What is Cyxodil used for:
Cyxodil is used to treat chronic asthma in adult and adolescent patients (12 years and older).
How Cyxodil works
Cyxodil helps to make breathing easier by reducing the severity of asthma symptoms and reducing the likelihood of an asthma attack. The effect of the medicine increases over time, so it should be taken daily, even if the patient feels better. This medicine is not suitable for use during an acute asthma attack. To quickly relieve such an attack, only a bronchodilator inhaler should be used.
2. Important information before using Cyxodil
When not to use Cyxodil:
Warnings and precautions
Before starting to use Cyxodil, discuss it with your doctor or pharmacist. Before startingtreatment with this medicine, inform your doctor if:
- the patient has ever been treated for or is currently being treated for tuberculosis, fungal, viral, or bacterial infections.
In case of doubts, consult your doctor. It is essential to ensure that Cyxodil is suitable for the patient. If, while using Cyxodil, the patient:
- experiences difficulty breathing and worsening symptoms, such as coughing, shortness of breath, wheezing, feeling of chest tightness, increased wheezing, or other symptoms of bronchial constriction. A bronchodilator inhaler should be used, which usually results in rapid improvement.
- experiences symptoms that wake them up from sleep;
- does not improve after using a bronchodilator inhaler. The doctor will determine further treatment for the patient.
Special patient groups
In patients with severe asthma, there is a risk of acute asthma attacks. In this patient group, the doctor will regularly perform thorough examinations to assess asthma control, including lung function tests. Patients who are already taking corticosteroids in tablets:
Cyxodil may be used instead of tablets or to reduce the number of tablets needed. Follow the doctor's instructions exactly.
- The replacement will start about a week after starting to use the Cyxodil inhaler.
- The number of tablets taken will be carefully reduced over a period.
- During this time, the patient may sometimes feel unwell.
- Despite this, it is essential to continue the Cyxodil inhaler treatment and gradually reduce the number of tablets taken.
- If severe symptoms occur, such as nausea, vomiting, diarrhea, or high fever, consult a doctor.
- Switching from tablets to inhalation may sometimes cause mild allergic reactions, such as nasal congestion or rash (itchy redness of the skin).
- After switching from tablets to inhalation, the patient may be at risk of reduced adrenal function for some time, related to the previously taken corticosteroids in tablets. Symptoms of reduced adrenal function (e.g., dizziness, fainting, nausea, loss of appetite, mood changes, hair loss, reduced resistance to stress, weakness, headaches, memory disorders, allergies, increased appetite, and changes in blood sugar levels) may also persist for some time.
- It may also be necessary to consult a specialist to determine the degree of reduced adrenal function.
- The doctor will also regularly monitor adrenal function.
- In stressful situations, such as during surgery, worsening of asthma attacks, additional corticosteroid tablets may be necessary. Therefore, the patient should carry a steroid warning card with this information.
Patients with liver or kidney disease
No dose adjustment of ciclesonide is necessary in patients with liver or kidney disease.
If the patient has severe liver disease, the doctor will carefully assess the risk of potential side effects due to impaired steroid production.
Children
This medicine is not recommended for use in children under 12 yearsof age, as there is no information on its potential effects in this age group.
Cyxodil and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or plan to take, including those available without a prescription.
Before using Cyxodil, inform your doctor if you are currently being treated for any fungal or viral infection with a medicine containing:
- ketokonazole,
- itraconazole,
- cobicistat,
- ritonavir,
- nelfinavir. These medicines may enhance the effect of Cyxodil, so it is not possible to completely rule out the possibility of side effects.
Cyxodil with food and drink
No interactions have been found between the use of Cyxodil and food or drink.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor or pharmacist before using this medicine.
- Since there is not enough information on the use of Cyxodil in pregnant women, the doctor will discuss the risks and benefits of using Cyxodil with the patient.
- Ciclesonide (the active substance of Cyxodil) may be used during pregnancy only if the potential benefits to the mother outweigh the possible risk to the developing child. If the doctor decides that the patient can continue to use Cyxodil, the smallest possible dose of ciclesonide will be used to maintain control of asthma.
- Adrenal function will be carefully monitored in children whose mothers received corticosteroids during pregnancy.
- If the patient plans to use Cyxodil while breastfeeding, they should discuss this with their doctor.
- It is not known whether inhaled ciclesonide passes into breast milk.
- Cyxodil will only be prescribed to breastfeeding women if the expected benefits to the mother outweigh the possible risk to the child.
Driving and using machines
Cyxodil and its excipients do not affect or have a negligible effect on the ability to drive or use machines.
Cyxodil contains ethanol
This medicine contains 4.7 mg of alcohol (ethanol) in each dose. The amount of alcohol in each dose of this medicine is equivalent to less than 1 ml of beer or 1 ml of wine. The small amount of alcohol in this medicine will not have noticeable effects.
3. How to use Cyxodil
Always use this medicine exactly as your doctor or pharmacist has told you. If you are not sure, ask your doctor or pharmacist.
- If you started using this medicine instead of corticosteroids in tablets or together with corticosteroids in tablets, see section 2 "Patients who are already taking corticosteroids in tablets".
What dose of Cyxodil should be used each day?
Your doctor will tell you what dose to use each day. This will depend on your individual needs.
- The recommended dose of Cyxodil is 160 micrograms once a day and is the dose that controls asthma symptoms in most patients.
- In some patients, a reduced dose of 80 micrograms once a day may be sufficient to maintain adequate asthma control.
- In patients with severe worsening of asthma symptoms, it may be necessary to temporarily increase the dose of Cyxodil. This may be up to 640 micrograms per day, given in doses of 320 micrograms twice a day, but there is no data to support additional therapeutic effects after 3 months of using higher doses.
If necessary, your doctor may also prescribe corticosteroids in tablets and/or antibiotics in case of infection.
- Your doctor will adjust the dose to the minimum necessary to control your asthma.
- Improvement in symptoms (wheezing, chest tightness, and coughing) should be noticeable within 24 hours.
When to use Cyxodil?
Usually, either in the morning or in the evening - one or two doses once a day. Follow your doctor's instructions exactly. It is essential to use Cyxodil regularly every day, even if you feel better.
If you find that you need to use a bronchodilator inhaler more than 2-3 times a week, you should consult your doctor, who will reassess your treatment.
How to use Cyxodil?
It is essential that your doctor, nurse, or pharmacist shows you how to use the Cyxodil inhaler correctly. Good technique will ensure that the correct dose of medicine is delivered to your lungs. The instructions in this leaflet should be used as a reminder.
To ensure that you are using the Cyxodil inhaler correctly, it may be necessary to perform a few initial doses in front of a mirror. Make sure that the medicine is not escaping from the top or sides of your mouth.
A new inhaler or one that has not been used for a week or longer mustbe checked before use. Remove the mouthpiece cover and press the canister threetimes to release threedoses, directing the spray into the air away from you.
Shaking the Cyxodil inhaler before use is notnecessary. The medicine is a uniform solution that ensures the correct dose is delivered to the patient during each inhalation.
Follow the instructions below exactly, using the accompanying diagrams.
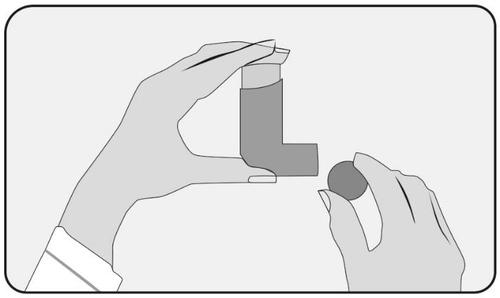
- 1. Remove the mouthpiece cover and check the mouthpiece inside and out to make sure it is clean and dry.
- 2. Hold the inhaler with the canister facing upwards (the base of the canister at the top), placing your index finger on the bottom of the canister and your thumb under the mouthpiece.
- 3. Breathe out as slowly and deeply as is comfortable. Do not breathe out into the inhaler.
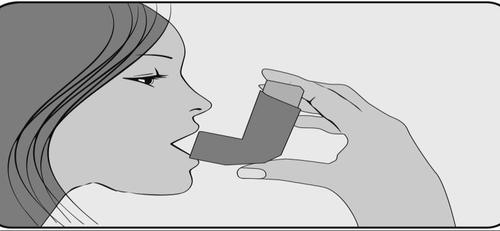
- 4. Put the mouthpiece in your mouth and close your lips around it, firmlyholding the mouthpiece.
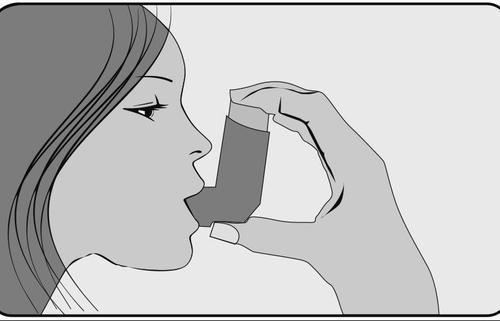
- 5. Just after starting to breathe in through your mouth, press your index finger on the top of the inhaler to release a dose of medicine, taking a slow and deep breath at the same time. Make sure that the dose of medicine is not escaping from the top, bottom, or sides of your mouth.
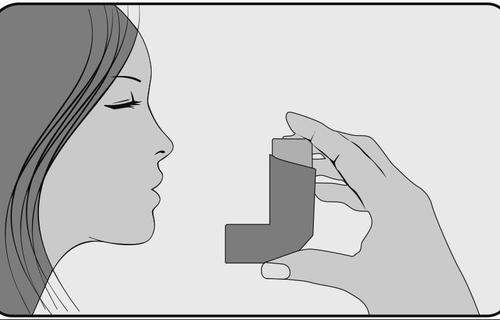
- 6. Hold your breath, remove the inhaler from your mouth, and take your index finger off the top of the inhaler. Continue to hold your breath for about 10 seconds or as long as is comfortable. Breathe out slowly through your mouth. Avoid blowing into the inhaler.
It is essential not to rush when performing the actions described in steps 3 to 6.
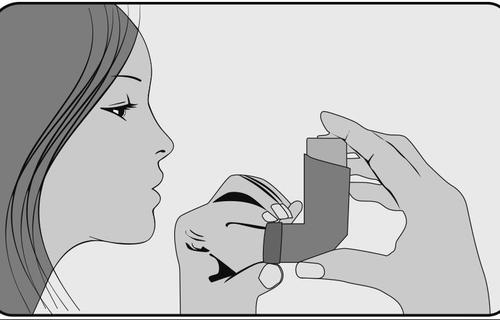
- 7. If a second dose is prescribed, waitfor about half a minute and repeatsteps 3 to 6.
- 8. After use, always replace the mouthpiece cover to prevent dust from entering. Press the cover firmly and snap it into place.
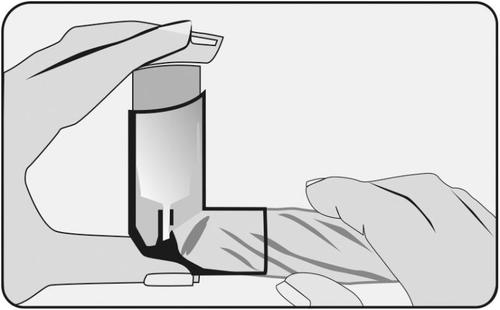
For hygiene reasons, the inner and outer surfaces of the mouthpiece should be cleaned with a drytissue every week.
- 1. Remove the mouthpiece cover, without removing the inhaler from the plastic casing;
- 2. Wipe the front of the small hole where the medicine is released, using a dryfolded tissue;
- 3. Do not use water or other liquids.
Proper technique will ensure that the correct dose of Cyxodil is delivered to the patient's lungs during each use of the inhaler. Your doctor will regularly check your inhalation technique to ensure the best treatment effect.
After the canister is completely empty, the spray will no longer be heard or felt.
If, after using Cyxodil, the patient experiences wheezing or chest tightness:
- do not use additional doses.
- use a bronchodilator inhaler to relieve breathing.
- consult your doctor immediately.
If using the inhaler is difficult for the patient, the doctor may recommend using a spacer. The spacer that fits the Cyxodil inhaler is called AeroChamber Plus Flow-Vu.
When using the AeroChamber Plus Flow-Vu device, follow the instructions provided with it.
Your doctor or pharmacist will advise on the use of this device.
Using a higher dose of Cyxodil than recommended
It is essential to use the dose of Cyxodil prescribed by your doctor. Do not increase or decrease the dose without consulting your doctor.
In case of overdose, there is no need for special treatment, but you should inform your doctor. When using high doses for an extended period, it is not possible to rule out the risk of reduced adrenal function, and monitoring may be necessary.
Missing a dose of Cyxodil
If you miss a dose of Cyxodil, take the next dose at the scheduled time. Do not take a double dose to make up for the missed dose.
Stopping the use of Cyxodil
Do not stop using the Cyxodil inhaler, even if you feel better.
If you stop using this medicine, tell your doctor immediately.
If you have any further doubts about using this medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Cyxodil can cause side effects, although not everybody gets them.
Stop using the medicine and consult your doctor immediately if you experience any of the following severe side effects:
- severe allergic reactions, such as swelling of the mouth, tongue, and throat (occurring in less than 1 in 1000 patients),
- allergic reactions: skin rash, redness, itching, or hives (occurring in less than 1 in 100 patients),
- coughing or wheezing worsening shortly after inhalation (occurring in less than 1 in 100 patients).
Other side effects that may occur when using Cyxodil are usually mild. In most cases, the patient can continue treatment. Side effects that may occur include:
- hoarseness
- burning, inflammation, or irritation of the mouth or throat
- thrush (fungal infection of the mouth)
- headache
- unpleasant taste in the mouth
- dryness in the mouth or throat
- nausea or vomiting.
Rare side effects (occurring in less than 1 in 1000 patients):
- heart palpitations (rapid heartbeat)
- discomfort or abdominal pain
- high blood pressure.
Side effects with unknown frequency, but also possible:
- sleep disturbances, depression, or feelings of worry, anxiety, nervousness, increased agitation, or irritability. The occurrence of these effects is more likely in children.
- blurred vision and eye diseases causing vision disturbances (central serous chorioretinopathy).
Cyxodil may affect the normal production of corticosteroids in the patient's body. This is usually observed in patients taking high doses for a long time. This effect may include:
- slowed growth in adolescents
- thinning of bones
- possible cataract (clouding of the lens of the eye) causing blurred vision
- vision loss due to abnormally high pressure in the eye (glaucoma)
- moon-shaped face, increased weight in the upper body, and thinning of the arms and legs (Cushing's syndrome). The doctor will regularly monitor the growth of adolescents receiving long-term treatment. If growth slows down, the doctor will adjust the dose to the smallest possible dose that maintains effective asthma control, if possible.
Corticosteroids in tablets may cause more side effects than inhaled corticosteroids, such as Cyxodil. In patients who have taken steroid tablets before or during the use of Cyxodil, the risk of side effects related to tablet use may persist for some time. Regular medical check-ups will ensure that the doses of Cyxodil used by the patient are appropriate. Regular check-ups will also allow for early detection of any side effects and reduce the likelihood of their worsening.
Remember that:
- If any of the side effects worsen or if you experience any side effects not listed in this leaflet, tell your doctor or pharmacist.
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, tell your doctor, pharmacist, or nurse.
Side effects can be reported directly to the Department of Post-Marketing Surveillance of Medicinal Products, Medical Devices, and Biocidal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Jerozolimskie Avenue 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Cyxodil
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and label after EXP. The expiry date refers to the last day of the month.
The inscription on the packaging after the abbreviation EXP indicates the expiry date, and after the abbreviation Lot, it indicates the batch number.
Pressurized container. Do not expose to temperatures above 50°C.
Do not pierce, damage, or burn, even if the canister appears to be empty.
Like most inhalation medicines in pressurized containers, the effectiveness of this medicine may be reduced if the canister is cold. However, Cyxodil delivers the same dose in a temperature range of -10°C to 40°C.
If the inhaler is very cold, before use, remove it from the plastic packaging and warm it in your hands for a few minutes. Never use anything else to heat it up.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Cyxodil contains:
- The active substance of Cyxodil is ciclesonide. Each actuation of the inhaler delivers a dose (delivered through the mouthpiece) that contains 80, 160, or 320 micrograms of ciclesonide.
- The other ingredients are ethanol anhydrous and norflurane (HFA-134a). This medicine contains fluorinated greenhouse gases. Each inhaler contains 8.8 g of HFA-134a, which corresponds to 0.013 tons of CO2 equivalent (GWP = 1430).
What Cyxodil looks like and what the pack contains
Cyxodil is a clear and colorless liquid in an aluminum pressurized canister that delivers a precisely measured dose of ciclesonide in the form of an aerosol.
The canister is placed in a plastic casing consisting of a mouthpiece with a green (Cyxodil 80 micrograms) or purple (Cyxodil 160 micrograms) or red (Cyxodil 320 micrograms) protective cap.
Pack sizes:
1 inhaler containing 120 metered doses.
Marketing authorization holder and manufacturer
Marketing authorization holder
Polpharma S.A.
Pelplińska Street 19, 83-200 Starogard Gdański
phone: +48 22 364 61 01
Manufacturer
Genetic S.p.A.
Contrada Canfora
84084 Fisciano (SA)
Italy
This medicine is authorized in the Member States of the European Economic Area under the following names:
Poland: Cyxodil
Czech Republic: Ciclesonide Polpharma
Date of last revision of the leaflet:February 2025
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterGenetic S.p.A
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CixodilDosage form: Aerosol, 160 mcg/dose inh.Active substance: ciclesonidePrescription requiredDosage form: Aerosol, 160 mcg/dose inh.Active substance: ciclesonidePrescription requiredDosage form: Aerosol, 80 mcg/dose inh.Active substance: ciclesonidePrescription required
Alternatives to Cixodil in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Cixodil in Украина
Alternative to Cixodil in Испания
Online doctors for Cixodil
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Cixodil – subject to medical assessment and local rules.














