
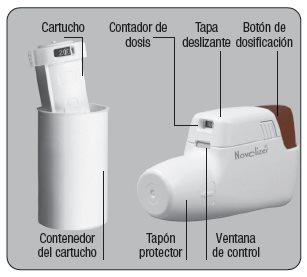
NOVOPULM NOVOLIZER 400 micrograms POWDER FOR INHALATION


How to use NOVOPULM NOVOLIZER 400 micrograms POWDER FOR INHALATION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What Novopulm Novolizer 400 micrograms is and what it is used for
- What you need to know before you use Novopulm Novolizer 400 micrograms
- How to use Novopulm Novolizer 400 micrograms
- Possible side effects
- Storing Novopulm Novolizer 400 micrograms
- Container Content and Additional Information
Introduction
Package Leaflet: Information for the User
Novopulm Novolizer 400 micrograms
Inhalation Powder
Budesonide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What Novopulm Novolizer 400 micrograms is and what it is used for.
- What you need to know before you use Novopulm Novolizer 400 micrograms.
- How to use Novopulm Novolizer 400 micrograms.
- Possible side effects.
- Storing Novopulm Novolizer 400 micrograms.
- Contents of the pack and further information.
1. What Novopulm Novolizer 400 micrograms is and what it is used for
Budesonide, the active substance of Novopulm Novolizer 400 micrograms, is an inhaled glucocorticoid (corticosteroid).
Novopulm Novolizer 400 micrograms is used for the regular treatment of persistent asthma.
NOTE:
Novopulm Novolizer 400 micrograms should not be used for the treatment of an acute asthma attack (status asthmaticus).
2. What you need to know before you use Novopulm Novolizer 400 micrograms
Do not use Novopulm Novolizer 400 micrograms
If you are allergic to budesonide or to the milk proteins that are contained in small amounts in the excipient lactose monohydrate (included in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Novopulm Novolizer 400 micrograms.
Contact your doctor if you experience blurred vision or other visual disturbances.
Be especially careful with Novopulm Novolizer 400 micrograms
If you have pulmonary tuberculosis or a fungal infection or other airway infection. This also applies if you have had these problems in the past. Inform your doctor.
Budesonide is not suitable for the treatment of an acute asthma attack or continuous severe bronchial spasms (status asthmaticus). Your doctor will instruct you on the use of short-acting inhaled bronchodilators (bronchodilator) as rescue medication for the relief of acute asthma symptoms.
If you have severe liver problems, the elimination of budesonide may be altered. This may lead to an increase in budesonide in the blood.
Any inhaled glucocorticoid may cause side effects, particularly when used in high doses for prolonged periods. These effects occur with less likelihood with inhaled treatment than with the administration of glucocorticoids in tablets. The possible effects include alteration of adrenal function, Cushing's syndrome, Cushingoid features (hormonal disorders caused by high levels of cortisol in the blood with central obesity, "moon face", thinning of the skin, hypertension, etc.), decreased bone density, growth retardation in children and adolescents, as well as eye disorders (cataracts and glaucoma), and more rarely a series of psychological and behavioral effects such as psychomotor hyperactivity, sleep disturbances, anxiety, depression or aggression (particularly in children). Therefore, it is important that the lowest dose is administered with which effective asthma control is maintained.
During the first few months after switching from tablet treatment to inhaled treatment, periods of stress or emergencies (e.g., severe infections, injuries, and surgery) may occur, and it may be necessary to resume systemic administration of glucocorticoids in the form of tablets or infusions. This also applies to patients who have received prolonged treatment with high doses of inhaled glucocorticoids. They may also have altered adrenal function, requiring coverage with systemic corticosteroids during periods of stress and/or elective surgery.
After switching to inhaled treatment, symptoms that had been suppressed by previous systemic glucocorticoid treatment may reappear, e.g., symptoms of allergic rhinitis, atopic eczema, or rheumatic symptoms. These symptoms should be treated additionally with appropriate medications.
Some patients may generally experience non-specific discomfort during the transition period, despite maintenance or even improvement of respiratory function. In such cases, consult your doctor. The doctor will decide whether the treatment can continue or be interrupted in case of, for example, symptoms of adrenal insufficiency.
Using Novopulm Novolizer 400 micrograms with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines, including those obtained without a prescription.
Some medicines may increase the effects of Novopulm Novolizer 400 micrograms, so your doctor will monitor you closely if you are taking these medicines (including some for HIV: nelfinavir, ritonavir, cobicistat, and medicines for the treatment of fungal infections: ketoconazole, itraconazole).
Consequently, this combination should be avoided. If this is not possible, the time interval between the administration of these medicines and budesonide should be as long as possible.
In women treated with estrogens and steroid contraceptives, elevated plasma concentrations and intensified effects of corticosteroids have been observed, but no effects have been observed with budesonide and the concomitant administration of low-dose oral contraceptives.
Since adrenal function may be suppressed, false results (low values) may appear in ACTH stimulation tests for the diagnosis of pituitary insufficiency.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Pregnancy
Most results from epidemiological studies and post-marketing data worldwide have not been able to detect an increased risk of adverse effects in the fetus or newborns after the use of inhaled budesonide during pregnancy. It is essential for both the mother and the fetus to maintain adequate asthma treatment during pregnancy.
As with other medicines administered during pregnancy, the benefit of administering inhaled budesonide for the mother should be weighed against the risks for the fetus.
.
Breastfeeding
Budesonide passes into breast milk. However, at therapeutic doses, no effects are expected in the breastfed infant. Maintenance treatment with inhaled budesonide (200 or 400 micrograms twice daily) in asthmatic women during breastfeeding results in negligible systemic exposure to budesonide in breastfed infants. Therefore, Novopulm Novolizer 400 micrograms can be used during breastfeeding.
Driving and using machines
Budesonide has no influence on the ability to drive and use machines.
Novopulm Novolizer 400 micrograms contains milk sugar (lactose), 10.5 mg of lactose monohydrate/dose released.
This medicine contains lactose. It may cause allergic reactions in patients with milk protein allergy. If your doctor has told you that you have an intolerance to some sugars, consult with them before using this medicine.
If your doctor has told you that you have an intolerance to some sugars, consult with them before using this medicine.
3. How to use Novopulm Novolizer 400 micrograms
Follow exactly the instructions for administration of this medicine indicated by your doctor. In case of doubt, consult your doctor or pharmacist.
Patients who start treatment with glucocorticoids and patients previously treated with inhaled glucocorticoids.
Unless your doctor tells you otherwise, the recommended dose is as follows:
Adults (including elderly people) and children/adolescents over 12 years:
Recommended initial dose: 1 single dose (400 micrograms) once or twice daily.
Recommended maximum dose: 2 single doses (800 micrograms) twice daily (daily dose: 1600 micrograms).
Children from 6 to 12 years:
Recommended initial dose: 1 single dose (400 micrograms) once daily.
Recommended maximum dose: 1 single dose (400 micrograms) twice daily (daily dose: 800 micrograms).
In case of administration once daily, the dose should be administered in the evening.
Children under 6 years:
The use of Novopulm Novolizer 400 micrograms is not recommended in children under 6 years of age due to insufficient safety and efficacy data.
Supervise the child for correct handling of the Novolizer device.
Children
In children receiving prolonged treatment with inhaled glucocorticoids, regular monitoring of growth is recommended.
Elderly
Normally, no special dose adjustments are required. In general, the lowest dose required for effective control should be used.
In case of deterioration of asthma symptoms (recognizable, for example, by persistent respiratory symptoms and increased use of an inhaled bronchodilator), you should consult your doctor as soon as possible. If you were receiving a single daily dose, you may now need the same dose but administered twice daily (in the morning and evening). In any case, your doctor will decide whether you need to increase your usual dose of Novopulm Novolizer 400 micrograms.
Tell your doctor or pharmacist if you think that the effect of Novopulm Novolizer 400 micrograms is too strong or too weak.
You should always carry a short-acting inhaled bronchodilator (beta-2-agonist, such as salbutamol) for the relief of acute asthma symptoms.
When switching from another budesonide inhaler to Novopulm Novolizer 400 micrograms, the treatment plan should be adjusted by your doctor.
Method of administration
Inhalation.
Follow the Instructions for Use to perform the inhalation.
Important information for handling
To reduce the risk of fungal infection in the mouth and throat (oral candidiasis) and hoarseness, it is recommended that inhalation be performed before meals and/or that the mouth be rinsed with water or teeth brushed after each inhalation.
Duration of treatment
Novopulm Novolizer 400 micrograms is intended for long-term therapy. It should be administered regularly, according to the recommended treatment schedule, even during periods when the patient is asymptomatic.
If you have never used glucocorticoids before or have only received them occasionally for short periods, regular use of Novopulm Novolizer 400 micrograms will make you experience an improvement in breathing after approximately 10 days. However, extreme mucous congestion and inflammatory processes may obstruct the bronchial tubes to the point where budesonide cannot fully exert its effects in the lung. In such cases, the initiation of therapy should be supplemented with the administration of cortisone tablets (systemic glucocorticoids). Subsequently, the dose of tablets should be gradually reduced but continuing with inhaled therapy.
If you have already used cortisone for prolonged periods, the switch to Novopulm Novolizer 400 micrograms should be made when your symptoms are completely controlled. Normally, in this situation, adrenal function is suppressed, and therefore, the intake of cortisone tablets (systemic corticosteroids) should be gradually reduced without abrupt interruption. At the start of the transition period, Novopulm Novolizer 400 micrograms should be administered simultaneously for approximately 10 days. Then, depending on your response, the daily dose of cortisone tablets can be gradually reduced at intervals of one to two weeks.
If you inhale more Novopulm Novolizer 400 micrograms than you should:
It is important that you use the dose indicated by your doctor or pharmacist. Do not increase or decrease the dose without consulting your doctor.
In case of overdose or accidental ingestion, consult the Toxicology Information Service, telephone (91) 562 04 20.
If you forget to use Novopulm Novolizer 400 micrograms
Do not take a double dose to make up for forgotten doses.
If you stop using Novopulm Novolizer 400 micrograms
Do not stop using Novopulm Novolizer 400 micrograms without consulting your doctor first, as this may lead to a worsening of your disease.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Most important side effects
Frequent side effects may include irritation of the oral mucosa (irritation of the throat), accompanied by difficulty swallowing, hoarseness, and cough.
Treatment with inhaled budesonide may result in infection of the mouth and throat by fungi (oropharyngeal candidiasis). Experience has shown that this fungal infection occurs less frequently when inhalation is performed before meals and/or when the mouth is rinsed with water or teeth brushed after inhalation. In most cases, this condition responds to topical antifungal therapy without interrupting treatment with Novopulm Novolizer 400 micrograms.
As with other inhalation therapies, in rare cases, bronchial spasm (paradoxical bronchospasm) may occur, which is manifested by a temporary increase in breathing difficulties and an immediate increase in wheezing after administration. Only in such cases should the use of Novopulm Novolizer 400 micrograms be discontinued without prior consultation, and then contact your doctor immediately.
Inhalation of large doses over a prolonged period may increase susceptibility to infections. The ability to adapt to stress may be altered.
Other side effects
Uncommon (may affect up to 1 in 100 people)
Depression, anxiety or feeling of worry, cataracts, muscle spasm, tremor, blurred vision.
Rare (may affect up to 1 in 1,000 people):
Allergic reactions (hypersensitivity) and swelling of the face, eyes, lips, mouth, and throat (angioedema), anaphylactic reaction;
suppression of adrenal function (adrenal suppression), growth retardation in children and adolescents;
restlessness, nervousness, abnormal behavior, over-excitement or irritability (these effects are more frequent in children); skin reactions such as rash (urticaria), eczema, localized skin inflammation (dermatitis), itching (pruritus), redness of the skin due to congestion of the capillaries (erythema), bruising, changes in voice and hoarse voice (in children).
Very rare (may affect up to 1 in 10,000 people):
Decreased bone density.
Frequency not known (cannot be estimated from the available data): Sleep disturbances, aggression, excessive activity accompanied by mental restlessness (psychomotor hyperactivity); glaucoma.
The lactose monohydrate contains small amounts of milk proteins and may therefore cause allergic reactions.
Reporting of side effects:
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Novopulm Novolizer 400 micrograms
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label, carton, and cartridge after EXP. The expiry date is the last day of the month stated.
Storage conditions
Store in the original package. No special storage temperature is required.
Storage conditions in use: Keep the Novolizer device perfectly closed to protect it from moisture.
Information on the period of validity
Replace the cartridge 6 months after the first opening.
Do not use the powder inhaler for more than 1 year.
Note: It has been demonstrated that the Novolizer device functions for at least 2000 single doses. Therefore, with this device, a maximum of 20 cartridges of 100 single doses and/or 40 cartridges of 50 single doses (during one year) can be used before replacing it.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and any unused medicine in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Container Content and Additional Information
Composition of Novopulm Novolizer 400 micrograms
The active ingredient is budesonide.
One inhalation (puff) contains 400 micrograms of budesonide.
The other component is lactose monohydrate.
Appearance of Novopulm Novolizer 400 micrograms and Container Content
Novopulm Novolizer 400 micrograms, inhalation powder, contains a white powder (0.545 g or 1.09 g) in a cartridge with 50 or 100 measured doses, packaged in a sealed container with an aluminum foil and a powder inhaler, Novolizer.
All components are made of plastic materials.
Package sizes:
Original Sales Packages:
1 cartridge with 50/100 measured doses and 1 powder inhaler, Novolizer.
2 cartridges with 100 measured doses each and 1 powder inhaler, Novolizer.
Replacement Packages:
1 cartridge with 50/100 measured doses
2 cartridges with 100 measured doses each.
Not all package sizes may be marketed
Marketing Authorization Holder:
Viatris Healthcare Limited
Damastown Industrial Park
Mulhuddart, Dublin 15
Dublin
Manufacturer:
McDermott Laboratories T/A Mylan Dublin Respiratory
Unit 25 Baldoyle Industrial Estate
Grange Road, Baldoyle
Dublin 13
Ireland
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Viatris Pharmaceuticals, S.L.
C/ General Aranaz, 86
28027 - Madrid
Spain
This medication is authorized in the member states of the European Economic Area and the United Kingdom (Northern Ireland) with the following names:
Austria
Novolizer Budesonid Meda 400 Mikrogramm Pulver zur Inhalation
Belgium and Luxembourg:
Novolizer Budesonide 400 microgrammes, poudre pour inhalation
France:
Novopulmon Novolizer 400 microgrammes/dose, poudre pour inhalation
Germany:
Novopulmonm 400 Novolizer, Pulver zur Inhalation
United Kingdom (Northern Ireland):
Budelin Novolizer 400 micrograms per actuation inhalation powder
Italy:
Budesonide Viatris Novolizer 400 microgrammi polvere per inalazione
Netherlands:
Budesonid Novolizer 400 µg, inhalatiepoeder
Portugal:
Budesonido Novolizer 400 microgramas pó para inalação
Spain:
Novopulm Novolizer 400 microgramos, polvo para inhalación
Date of the last revision of this prospectus: 09/2022
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
I N S T R U C T I O N S F O R U S E
Novolizer
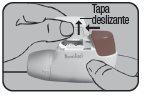
- PREPARATION:
The Dry Powder Inhaler Novolizer makes inhalation a simple and reliable process. Its direct use, quick cartridge replacement, and easy cleaning are carried out in a simple and fast manner.
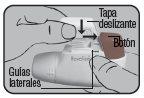
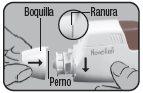
Place the Dry Powder Inhaler Novolizer in front of you. Press the rough surfaces on both sides of the cap, slide the cap forward, and lift it.
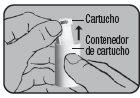
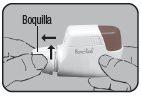
 Remove the aluminum protective foil from the cartridge container and extract the new cartridge. This operation should only be performed immediately before using the cartridge. The color coding of the cartridge must match the color of the dosing button.
Remove the aluminum protective foil from the cartridge container and extract the new cartridge. This operation should only be performed immediately before using the cartridge. The color coding of the cartridge must match the color of the dosing button.
First cartridge:
Insert the cartridge into the Dry Powder Inhaler Novolizer with the dose counter oriented towards the mouthpiece. Do not press the dosing button during cartridge insertion.
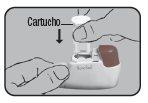
 Replacement:
Replacement:
Note: The Dry Powder Inhaler Novolizer should be cleaned each time the cartridge is changed, after removing the empty cartridge.
If you have already used the Dry Powder Inhaler Novolizer, first remove the empty cartridge and then insert the new one. Do not press the dosing button during cartridge insertion.
 Place the cap back on its lateral guides from above and push it down towards the colored dosing button until it clicks into place.
Place the cap back on its lateral guides from above and push it down towards the colored dosing button until it clicks into place.
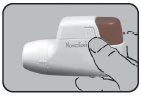
 Now, Novolizer is loaded and ready to be used.
Now, Novolizer is loaded and ready to be used.
You can leave the cartridge in the Dry Powder Inhaler Novolizer until it is empty or for up to 6 months after insertion. The cartridge is empty if a "0" is visible in the middle of the dose counter. A new cartridge should then be inserted. Cartridges can only be used in the original dry powder inhaler.
- USE:
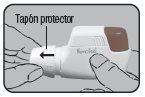
Whenever possible, stand or sit during inhalation. When using Novolizer, always keep it in a horizontal position. First, remove the protective cap.
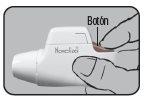
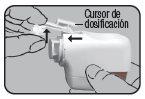
Press the colored dosing button completely. A double audible click will be heard, and the color of the control window will change from red to green. Then, release the colored button. The green color in the control window indicates that Novolizer is ready to be used.
Exhale (but not over the Dry Powder Inhaler Novolizer). Place your lips around the mouthpiece, inhale the powder constantly, deeply, and as quickly as possible (until maximum inhalation), and hold your breath for a few seconds. During this inspiration, an audible click should be heard, indicating a correct inhalation. Then, continue breathing normally.
Check that the color of the control window has changed back to red, which also indicates a correct inhalation. Replace the protective cap over the mouthpiece. This completes the inhalation procedure.
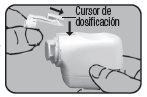
The figure appearing in the upper window indicates the number of inhalations remaining. The numerical scale 200 (respectively 100)-60 is shown in steps of 20, and between 60 (respectively 50)-0, it is shown in steps of 10. If the click sound is not heard and the color does not change, repeat the procedure as described above.
NOTE: The colored dosing button should only be pressed immediately before inhalation.
With Novolizer, it is not possible to accidentally overdose. The audible click and the color change in the control window indicate that the inhalation has been carried out correctly. If the color of the control window does not change back to red, the inhalation should be repeated. If the inhalation is not performed correctly after several attempts, consult your doctor.
- CLEANING:
The Dry Powder Inhaler Novolizer should be cleaned at regular intervals, but at least every time the cartridge is changed.
Remove the protective cap and mouthpiece.
First, remove the protective cap. Then, hold the mouthpiece and briefly turn it counterclockwise until it comes loose. Then, extract it.
 Cleaning
Cleaning
Now, turn the Novolizer upside down. Hold the loose dose counter and slide it forward and up. Any powder residue can be removed by gently tapping.
Clean the mouthpiece, dose counter, and powder inhaler with a soft, dry cloth without threads.
DO NOT use water or detergent.
 Assembly – Insertion of the dose counter
Assembly – Insertion of the dose counter
After cleaning, insert the dose counter by sliding it down at an angle and pressing it to click into place.
Put the inhaler back in its normal position.
 Assembly – Attachment of the mouthpiece and protective cap
Assembly – Attachment of the mouthpiece and protective cap
Insert the mouthpiece with the pin on the left side of the upper slot and turn it to the right until it clicks into place. Finally, put the protective cap back on.
Notes
- The Prospectus describes the functioning of the medication. Please read it carefully in its entirety before using the inhaler for the first time.
- Novolizer, which is presented with various active substances, does not use any propellant and is designed to be reloaded. This makes Novolizer a very environmentally friendly product.
- It is not possible to overdose with Novolizer. Even pressing the button several times, there is no more powder available for inhalation. Press the button only when you are actually going to inhale. If you do not manage to inhale correctly after several attempts, consult your doctor.
- Novolizer can be reloaded using new cartridges* that contain the active substance, making it especially suitable for long-term use (up to one year).
- Do not shake the filled Novolizer.
- Please teach your children the correct handling of the device.
- Make sure your Novolizer is protected from moisture and heat and remains clean at all times.
- Regarding the corresponding medications, please consult your doctor.
Date of last revision: 09/2022
- Country of registration
- Average pharmacy price24.52 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NOVOPULM NOVOLIZER 400 micrograms POWDER FOR INHALATIONDosage form: PULMONARY INHALATION, 0.25 mg/mlActive substance: budesonideManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: PULMONARY INHALATION, 0.5 mg/mlActive substance: budesonideManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: PULMONARY INHALATION, 0.04000 gActive substance: budesonideManufacturer: Laboratorio Aldo Union S.L.Prescription required
Online doctors for NOVOPULM NOVOLIZER 400 micrograms POWDER FOR INHALATION
Discuss questions about NOVOPULM NOVOLIZER 400 micrograms POWDER FOR INHALATION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















