
ALVESCO 160 micrograms/INHALATION SOLUTION FOR INHALATION IN A PRESSURIZED CONTAINER

How to use ALVESCO 160 micrograms/INHALATION SOLUTION FOR INHALATION IN A PRESSURIZED CONTAINER
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Alvesco 160 micrograms/inhalation, inhalation solution in a pressurized container
Ciclesonide
Read the entire package leaflet carefully before starting to use the medication,as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Alvesco and what is it used for
- What you need to know before taking Alvesco
- How to take Alvesco
- Possible side effects
- Storage of Alvesco
- Contents of the pack and further information
1. What is Alvesco and what is it used for
What is Alvesco?
Alvesco is a clear, colorless inhalation aerosol to be inhaled by the mouth into the lungs. It is a preventive medication (corticosteroid) that must be taken daily and is only active when inhaled into the lungs.
The active substance in this medication is Ciclesonide (To consult other components, see section 6).
What is Alvesco used for?
Alvesco is used to treat persistent asthma in adults and adolescents 12 years of age and older.
How does Alvesco work?
Alvesco helps you breathe better by reducing asthma symptoms and the likelihood of an asthma attack. The effect of the medication increases over time, and for this reason, it should be taken daily, even when you feel well.
This medication is not suitable for asthma attacks. Use your rescue inhaler to quickly relieve these attacks.
2. What you need to know before taking Alvesco
Do not use Alvesco
if you are allergic to ciclesonide or any of the other components of this medication (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Alvesco.
Be careful with Alvesco
- Before starting treatmentwith this medication, inform your doctor if:
You have received or are receiving treatment for pulmonary tuberculosis and fungal, viral, or bacterial infections.
Consult your doctor if you have any doubts. It is essential to ensure that Alvesco is the right medication for you.
During treatment with Alvesco, go immediatelyto your doctor if:
- you start having difficulty breathing and some of your symptoms worsen: cough, shortness of breath, wheezing, chest tightness, noises (rhonchi), or other symptoms of airway constriction. (You should use your rescue inhaler, which will normally give you quick relief).
- if you wake up at night with symptoms.
- if you do not improve despite using the rescue inhaler.
Your doctor will decide on continuing treatment.
If you experience blurred vision or other visual problems, contact your doctor.
Special patient groups
Patients with severe asthmaare at risk of suffering asthma attacks. For these patients, the doctor will regularly monitor the asthma and even perform a lung function test.
Patients taking oral corticosteroids:
Alvesco can be used to replace oral corticosteroids or to reduce the number of tablets taken. Follow your doctor's instructions carefully.
- This situation can occur after approximately 1 week after starting inhalations with Alvesco.
- The number of tablets will be reduced with caution over a period of time.
- During this period, you may sometimes experience a general feeling of discomfort.
- Still, it is advisable to continue inhaling Alvesco and gradually reducing the number of tablets.
- If you experience severe symptoms such as nausea, vomiting, diarrhea, or fever, go to your doctor.
- This process may lead to minor allergic reactions such as rhinitis (inflammation of the nose) or eczema (itching and redness of the skin).
- If you have changed your tablet treatment, you will still be exposed to the risk of suffering from adrenal insufficiency for a while. This risk is related to the intake of oral corticosteroids. The symptoms of adrenal insufficiency (dizziness, fainting, nausea, loss of appetite, mood changes, hair loss, inability to cope with stress, weakness, headaches, memory problems, allergies, anxiety to eat, and blood glucose disorders) may persist for a while.
- You may need to see a specialist to determine the degree of reduction in adrenal function.
- Your doctor will also perform regular checks on adrenal function.
- During periods of stress, such as surgical interventions or worsening of asthma attacks, you may need to take additional oral corticosteroids. If so, you should carry an identification card indicating this intake.
Patient with liver or kidney disorders
If you have liver or kidney disorders, it is not necessary to adjust the dose of ciclesonide.
If you have severe liver disorders, your doctor will examine you more closely to see if there are any side effects resulting from an alteration in the normal production of steroids.
Childrenunder 12 years:
This medication is not recommended for children under 12years due to the lack of information on its possible side effects.
Other medications and Alvesco
Inform your doctor before using Alvesco if you are receiving treatment for a fungal or viral infection with medications containing:
- ketoconazole
- itraconazole
- ritonavir
- nelfinavir
These compounds may increase the effect of Alvesco, making it impossible to completely avoid the appearance of side effects.
Inform your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
Using Alvesco with food and drinks
Alvesco can be taken with or without a drink and food.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
- Your doctor will inform you of the risks and benefits of using Alvesco, as there is not enough data on the effects of Alvesco in pregnant women.
- Alvesco can be used during pregnancy only when the potential benefits to the mother outweigh the potential risks to the fetus. If your doctor decides that you can continue using Alvesco, you should use the minimum dose of ciclesonide necessary to maintain control of asthma.
- In children of mothers treated during pregnancy with corticosteroids, adrenal function will be carefully checked.
- Consult your doctor if you want to use Alvesco during breastfeeding.
- It is not known if inhaled ciclesonide passes into breast milk.
- The use of Alvesco in breastfeeding women will only be considered if the expected benefits to the mother outweigh the potential risks to the child.
Consult your doctor or pharmacist before using any medication.
Driving and using machines
There are no known effects of Alvesco on the ability to drive and use machines.
Alvesco contains ethanol
This medication contains 4.7 mg of alcohol (ethanol) per dose. The amount in a dose of this medication is equivalent to less than 1 ml of beer or wine. The small amount of alcohol in this medication does not produce any noticeable effect.
3. How to use Alvesco
Follow the instructions for administration of this medication indicated by your doctor or pharmacist exactly. If in doubt, consult your doctor or pharmacist again.
- If you have just started using this medication instead of or in addition to oral corticosteroids, see section 2, Patients taking oral corticosteroids.
How much Alvesco should I use daily?
Your doctor will have informed you of the amount of medication you need to use daily. This amount will be determined based on your needs.
- The recommended dose of Alvesco is 160 micrograms daily in a single dose. This dose relieves asthma in most patients.
- In some patients, a reduction in dose to 80 micrograms daily in a single dose may be adequate to maintain effective control of asthma.
- In some patients with severe worsening of asthma symptoms, it may be necessary to increase the dose of Alvesco for a short period. This dose can be up to 640 micrograms daily, divided into two doses of 320 micrograms each, although there is no data to confirm the additional therapeutic effect after three months of treatment with this dose.
If necessary, your doctor may prescribe oral corticosteroids or, in the case of an infection, an antibiotic.
- Your doctor will adjust your dose to the minimum necessary to control asthma.
- You should start to notice an improvement in symptoms (wheezing, chest tightness, and cough) within 24 hours.
When should I use my Alvesco inhaler?
In most cases, in the morning or afternoon, in one or two inhalations once a day. Follow your doctor's instructions carefully. It is essential to use Alvesco regularly every day, even when you feel better.
If you notice that you need to use your rescue inhaler more than two or three times a week, you should go to your doctor to review your treatment.
How do I use the Alvesco inhaler?
It is essential that a doctor, nurse, or pharmacist teaches you how to use the inhaler correctly first. Good technique will ensure that the amount of Alvesco that reaches your lungs is adequate. Follow the instructions in this package leaflet to remember its use.
It is advisable to practice in front of a mirror the first two times until you are sure you are using the Alvesco inhaler correctly. You mustensure that no amount of medication is lost through the top or sides of the mouth.
If you have a new inhaler, or if you have not used your inhaler for a week or more, you musttest it before use. Remove the mouthpiece cap and, with the inhaler away from you, press threetimes the cartridge inside the inhaler to perform threedischarges into the air.
Do notshake the Alvesco inhaler before use. The medication is in a suitable solution that guarantees the reception of the correct dose with each inhalation.
During inhalation, you can remain seated or standing.
Follow these instructions carefully and use the images to guide you.
- Remove the mouthpiece cap and check the mouthpiece, inside and out, to ensure it is clean and dry.

- Hold the inhaler upside down (the base of the cartridge towards you) with your index finger on the base of the cartridge and your thumb under the mouthpiece.

- Exhalethe air from your lungs as much as possible. Do not exhale into the inhaler.
- Place the mouthpiece in your mouth and close your lips firmlyaround it.

- Immediately after starting to inhale through your mouth, press the top of the inhaler with your index finger to make a discharge while continuing to inhale slowly and deeply. Be careful not to let the medication escape through the top or sides of the mouth.
- Hold your breath, remove the inhaler from your mouth, and your index finger from the top of the inhaler. Continue holding your breath for approximately 10 seconds or until you start to feel uncomfortable holding your breath. Exhale slowly through your mouth, but do not exhale into the inhaler.
It is advisable not to rush through steps 3 to 6

- If you have been instructed to perform another discharge, wait approximately half a minute and repeat steps 3 to 6.
- After use, always replace the mouthpiece cap to prevent dust from entering. When you close it properly, you will hear a click, indicating that it is closed correctly.

- For hygiene reasons:
- Clean the mouthpiece weekly with a dry cloth, inside and out.
- Use a dry, folded cloth, and clean the front of the small hole through which the medication comes out.
- Do notuse water or other liquids.
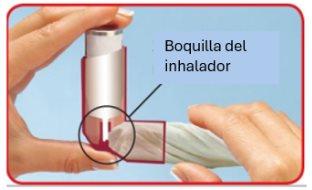
Good technique will ensure that the amount of medication that reaches your lungs is adequate each time you use the inhaler. Your doctor will check that you are using an adequate inhalation technique to ensure that the treatment can have its maximum effect.
When the cartridge is completely empty, you will not hear or feel the discharge of the propellant gas.
If you start having difficulty breathing or feel chest tightness after using the Alvesco inhaler:
- do not perform more discharges.
- use your rescue inhaler to help you breathe.
- inform your doctor immediately.
If you have difficulty using the inhaler, your doctor may recommend using a spacer device. The spacer device compatible with the Alvesco inhaler is called AeroChamber Plus. If you use the AeroChamber Plus device, follow its instructions for use. Your doctor or pharmacist can advise you on the use of this device.
If you use more Alvesco than you should
It is essential to take your dose according to your doctor's instructions. Do not increase or decrease your dose without consulting your doctor.
No specific treatment is necessary in case of using more Alvesco than you should, although you should inform your doctor. If you use high doses for extended periods, it cannot be ruled out that some degree of reduction in adrenal function may occur, and it may be necessary to monitor adrenal function.
If you forget to use Alvesco
If you forget to use the Alvesco inhaler, use the usual dose at the next intake. Do notperform a double discharge of the usual dose.
If you stop treatment with Alvesco
Even if you feel better, do not stop using your Alvesco inhaler.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
If you stop using Alvesco, you must inform your doctor immediately.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
If you consider that any of the adverse effects you are suffering from is serious or if you notice any adverse effect not mentioned in this prospectus, inform your doctor or pharmacist.
- Severe allergic reactions such as: swelling of the lips, tongue, and throat (may affect 1 in 1,000 patients treated)
- Allergic reactions: skin rash, redness, itching, or hives (may affect up to 1 in 1,000 patients treated, 1 in 100 patients)
- Cough or wheezing may worsen after inhalation (may affect 1 in 100 patients treated)
The rest of the adverse effects observed with Alvesco are usually mild. In most cases, treatment can be continued. The adverse effects you may experience are:
Uncommon adverse effects (may affect up to 1 in 100 patients treated):
- hoarseness,
- burning, inflammation, irritation of the mouth or throat
- oral thrush (fungal infections in the oral cavity)
- headache
- bad taste in the mouth
- dryness of the mouth or throat
- nausea or vomiting
Rare adverse effects (may affect up to 1 in 10,000 patients treated):
- heart palpitations
- abdominal discomfort or pain
- high blood pressure
Adverse effects of unknown frequency, but which may also occur:
- sleep disorders, depression, or feeling worried, restless, nervous, overexcited, or irritable. These effects are more likely in children
- blurred vision
Alvesco may affect the normal production of corticosteroids in your body. This is usually detected in patients who take high doses for a long period. Among these uncommon effects are:
- growth retardation in adolescents
- loss of bone density
- possible cataract (causing blurred vision)
- vision loss caused by excessively high eye pressure (glaucoma)
- moon face, weight gain in the trunk, and thinning of arms and legs (Cushing's syndrome or Cushingoid syndrome).
The doctor should regularly check the height of adolescents treated for a long period. If the growth rate decreases, your doctor will adjust the treatment to the lowest dose capable of maintaining effective control of asthma, if possible.
Oral corticosteroids can cause more adverse effects than inhaled ones like Alvesco. If you have taken oral corticosteroids before or during treatment with Alvesco, the risk of suffering from adverse effects related to taking these tablets may persist for some time. If you have regular check-ups, your doctor should check if you are taking the correct dose of Alvesco. In these consultations, adverse effects can be identified at an early stage, and the possibilities of them worsening can be reduced.
Remember that:
If you consider that any of the adverse effects you are suffering from is serious or if you notice any adverse effect not mentioned in this prospectus, inform your doctor or pharmacist.
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that does not appear in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Alvesco
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date that appears on the packaging, after EXP. The expiration date is the last day of the month indicated.
The packaging contains a pressurized liquid. Do not store at temperatures above 50°C.
Do not puncture, break, or burn the packaging, even when it appears to be empty.
Like all pressurized inhalers, the therapeutic effect of this medicine may decrease if the packaging is cold. However, Alvesco releases a constant dose within a temperature range of 10°C below zero and 40°C.
If your doctor decides to interrupt treatment or if the inhaler is empty, return it to the pharmacist so that it can be disposed of safely. This is important because there may be remaining medicine in the packaging, even if it appears to be completely empty. Do not puncture, break, or burn the packaging, even when it appears to be empty.
Medicines should not be thrown down the drain or into the trash. Deposit the packaging and medicines you no longer need at the SIGRE collection point in the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. In this way, you will help protect the environment.
6. Packaging Contents and Additional Information
Composition of Alvesco
- The active ingredientis ciclesonide. Each inhalation (dose released by the inhaler) contains 160 micrograms of ciclesonide.
- The other components (excipients) areanhydrous ethanol and a propellant gas (HFA-134a, Norflurano).
- This medicine contains fluorinated greenhouse gases.
Alvesco 160 micrograms 30 inhalations - Each inhaler contains 4.31 g of HFA-134a, which corresponds to 0.006 tons of CO2 equivalent (global warming potential GWP = 1430).
Alvesco 160 micrograms 60 inhalations - Each inhaler contains 5.59 g of HFA-134a, which corresponds to 0.008 tons of CO2 equivalent (global warming potential GWP = 1430).
Alvesco 160 micrograms 120 inhalations - Each inhaler contains 8.80 g of HFA-134a, which corresponds to 0.013 tons of CO2 equivalent (global warming potential GWP = 1430).
Appearance of Alvesco and Packaging Contents
Alvesco consists of a clear, colorless liquid in a pressurized aluminum packaging that releases a precise dose of ciclesonide in the form of an aerosol through the mouthpiece.
Packaging Sizes:
Inhaler with 30 inhalationsmeasured with precision
Inhaler with 60 inhalationsmeasured with precision
Inhaler with 120 inhalationsmeasured with precision
Each inhaler contains a sufficient dose for 30, 60, or 120 inhalations. Depending on the number of daily inhalations recommended by the doctor:
- the inhaler with 30 inhalations contains enough medicine for four weeks.
- the inhaler with 60 inhalations contains enough medicine for one or two months.
- the inhaler with 120 inhalations contains enough medicine for two or four months.
Only some packaging sizes may be marketed.
Marketing Authorization Holder and Manufacturer:
Marketing Authorization Holder
Covis Pharma Europe B.V.
Gustav Mahlerplein 2
1082 MA Amsterdam
Netherlands
Manufacturer:
Covis Pharma Europe B.V.
Gustav Mahlerplein 2
1082 MA Amsterdam
Netherlands
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Zentiva Spain S.L.U.
Paseo Club Deportivo 1, Edificio 4, Pozuelo de Alarcón 28223, Madrid
Spain
This medicine is authorized in other EU countries under the following names:
Austria | Alvesco 160, 80 Mikrogramm – Dosieraerosol |
Bulgaria | Alvesco 160, 80 micrograms, pressurised inhalation, solution |
Cyprus | Alvesco 160, 80 μικρογραμμ?ρια, δι?λυμα για εισπνο? υπ? π?εση |
Croatia | Alvesco 160, 80 mikrograma stlaceni inhalat, otopina |
Czech Republic | Alvesco 160 mikrogramu/dávka roztok k inhalaci v tlakovém obalu |
Denmark | Alvesco 160, 80 mikrogram/dosis inhalationsspray, opløsning |
Finland | Alvesco 160, 80 mikrog/annos inhalaatiosumute, liuos |
France | Alvesco 160, 80 microgrammes/dose, solution pour inhalation en flacon pressurisé |
Germany | Alvesco 160, 80 Mikrogramm Druckgasinhalation, Lösung |
Greece | Alvesco 160, 80 μικρογραμμ?ρια, δι?λυμα για εισπνο? υπ? π?εση |
Hungary | Alvesco 160, 80 mikrogramm túlnyomásos inhalációs oldat |
Ireland | Alvesco 160, 80 micrograms pressurised inhalation, solution |
Italy | Alvesco 160, 80 mcg soluzione pressurizzata per inalazione |
Latvia | Alvesco 160, 80 mikrogrami aerosols inhalacijam, zem spiediena, škidums |
Netherlands | Alvesco 160, 80 Inhalator, aërosol, oplossing 160, 80 microgram/dosis |
Norway | Alvesco 160, 80 mikrogram/dose inhalasjonsaerosol, oppløsning |
Poland | Alvesco 160, 80 160, 80 μg/dawke inhalacyjna; aerozol inhalacyjny, roztwór |
Romania | Alvesco 160, 80 Inhaler, 160, 80 micrograme/doza, solutie de inhalat presurizata |
Slovakia | Alvesco 160, 80 Inhalátor inhalacný roztok v tlakovom obale |
Slovenia | Alvesco 160, 80 mikrogramov/odmerek inhalacijska raztopina pod tlakom |
Spain | Alvesco 160 micrograms/inhalation solution for inhalation in a pressurized container |
Sweden | Alvesco 160, 80 microgram/dos inhalationsspray, lösning |
United Kingdom (Northern Ireland) | Alvesco 160, 80 Inhaler |
Date of the last revision of this prospectus:October 2024
Detailed and updated information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price49.17 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ALVESCO 160 micrograms/INHALATION SOLUTION FOR INHALATION IN A PRESSURIZED CONTAINERDosage form: PULMONARY INHALATION, 200 mcgActive substance: mometasoneManufacturer: Organon Salud S.L.Prescription requiredDosage form: PULMONARY INHALATION, 400 µgActive substance: mometasoneManufacturer: Organon Salud S.L.Prescription requiredDosage form: PULMONARY INHALATION, 100 micrograms/actuationActive substance: beclometasoneManufacturer: Laboratorio Aldo Union S.L.Prescription required
Online doctors for ALVESCO 160 micrograms/INHALATION SOLUTION FOR INHALATION IN A PRESSURIZED CONTAINER
Discuss questions about ALVESCO 160 micrograms/INHALATION SOLUTION FOR INHALATION IN A PRESSURIZED CONTAINER, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















