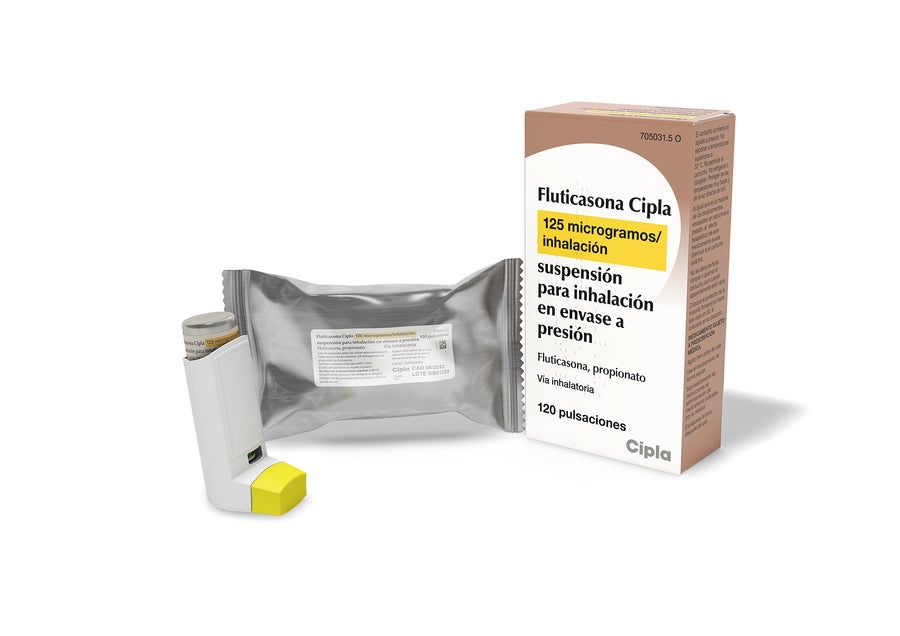
FLUTICASONE CIPLA 125 micrograms/inhalation suspension for inhalation in pressurized container

Ask a doctor about a prescription for FLUTICASONE CIPLA 125 micrograms/inhalation suspension for inhalation in pressurized container

How to use FLUTICASONE CIPLA 125 micrograms/inhalation suspension for inhalation in pressurized container
Introduction
Patient Information Leaflet
Fluticasona Cipla 125 micrograms/inhalation suspension for inhalation in a pressurized container
Fluticasona, propionate
Read this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Fluticasona Cipla and what is it used for
- What you need to know before you start using Fluticasona Cipla
- How to use Fluticasona Cipla
- Possible side effects
- Storage of Fluticasona Cipla
- Contents of the pack and further information
1. What is Fluticasona Cipla and what is it used for
This medicine contains the active substance fluticasone propionate, which belongs to a group of medicines called corticosteroids.
Fluticasona Cipla works by reducing inflammation in the lungs. This helps to prevent asthma attacks in patients who need regular treatment. This medicine takes between 4 to 7 days to start working, so it is very important that you use it regularly.
Fluticasona Cipla is not indicated for the treatment of acute asthma attacks where sudden shortness of breath occurs. In such cases, another medicine will be needed to treat the acute attacks.
2. What you need to know before you start using Fluticasona Cipla
Do not use Fluticasona Cipla:
- if you are allergic to fluticasone propionate or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Stop using Fluticasona Cipla immediately
- if you experience difficulty breathing with an immediate increase in wheezing just after taking a dose of this medicine.
Talk to your doctor, pharmacist, or nurse before you start using Fluticasona Cipla:
- if you have ever received treatment for tuberculosis (TB),
- if you have a history of diabetes mellitus (since fluticasone may increase blood glucose levels).
- if you have used high doses of this medicine for a long period of time and experience the following symptoms:
- weight gain and rounding of the face (moon face) (Cushing's syndrome),
- vague symptoms such as abdominal pain, nausea, diarrhea, headache, or drowsiness (suppressed adrenal function, acute adrenal crisis). These symptoms are more likely during an infection, such as viral infections or stomach upset,
- loss of bone mass,
- eye problems (cataracts and glaucoma),
- growth retardation (this occurs mainly in children and adolescents).
If you are not sure if any of the above applies to you, talk to your doctor, nurse, or pharmacist before using Fluticasona Cipla.
Contact your doctor if you experience blurred vision or other visual disturbances.
Using Fluticasona Cipla with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines, including those obtained without a prescription. In particular, tell your doctor or pharmacist if you are taking any of the following medicines:
- medicines used to treat various types of infections, such as ketoconazole, clarithromycin, telithromycin, atazanavir, indinavir, nelfinavir, or saquinavir. corticosteroid tablets, together with the Fluticasona Cipla inhaler or have just finished a course of corticosteroid tablets. You should carry a steroid treatment card, as there is a possibility that you may experience adrenal gland problems, especially during stressful situations such as a serious accident or if you are about to have an operation, and your doctor may decide to give you extra corticosteroids for a while.
- Some medicines may increase the effects of Fluticasona Cipla and your doctor may want to monitor you closely if you are taking these medicines (including some medicines for HIV: ritonavir, cobicistat)
If you are not sure if any of the above applies to you, talk to your doctor or pharmacist before taking Fluticasona Cipla.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
It is unlikely that Fluticasona Cipla will affect your ability to drive or use tools or machines.
3. How to use Fluticasona Cipla
Fluticasona Cipla is available in two different dose presentations for inhalation use. Your doctor will decide which dose of the medicine you need. Follow your doctor's instructions for using this medicine exactly. If you are in doubt, consult your doctor, nurse, or pharmacist again.
Using this medicine
- Fluticasona Cipla can be used with a Volumatic spacer device in the case of patients who have difficulty releasing a dose (puff) of the medicine just after starting to inhale.
- The initial dose will be the one indicated according to the severity of the disease. Your doctor will monitor your treatment to reduce the dose to the lowest dose that effectively controls your asthma.
Adults and adolescents over 16 years:
The recommended dose is 50 to 500 micrograms twice a day.
Use in children
The use of Fluticasona Cipla is not recommended in children under 16 years.
If you are using high doses of inhaled corticosteroids for a long period of time, you may need more corticosteroids, for example, during stressful situations such as a traffic accident or before surgery. Your doctor may decide to prescribe you extra corticosteroids during these situations.
Instructions for use
Your doctor, nurse, or pharmacist should teach you how to use your inhaler. They should check how you use it from time to time. Improper use of Fluticasona Cipla or not using it as prescribed may result in your asthma not improving as it should.
The medicine is packaged in a pressurized cartridge, inside a plastic casing with a mouthpiece. To avoid clogging the inhaler, it is essential that you clean it at least once a week.
Checking the inhaler
- Before using the inhaler for the first time, check that it is working properly. Remove the mouthpiece cover by gently squeezing the sides with your thumb and index finger and pulling it off.
- To check that the inhaler is working correctly, shake it well, point the mouthpiece away from you, and press the cartridge to release four puffs into the air. When you have not used the inhaler for a week or for a longer period, release two puffs into the air.
Using the inhaler
Just before using the inhaler, start breathing in as slowly as possible.
- You can do this standing or sitting.
- Remove the mouthpiece cover. Inspect the inside and outside of the mouthpiece to make sure it is clean and free of particles (Figure A).
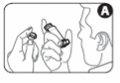
3 Shake the inhaler 4 or 5 times to ensure the removal of any foreign particles and that the contents of the inhaler are properly mixed (Figure B).

4 Hold the inhaler in a vertical position with your thumb at the base, below the mouthpiece. Expel as much air from your lungs as possible (Figure C). Do not inhale yet.
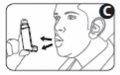
4 Place the mouthpiece of the inhaler between your teeth, and close your lips around the mouthpiece. Do not bite it (see Figure D).
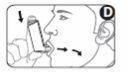
5 Inhale through your mouth and, just after starting to breathe in, press the top of the cartridge to release a dose of the medicine. Continue breathing in deeply and steadily (Figure D).
7 Hold your breath, remove the inhaler from your mouth, and remove your finger from the top of the inhaler. Continue holding your breath for a few seconds or as long as possible (Figure E).

8 If your doctor has told you to take two inhalations, wait about half a minute before taking another inhalation and repeat steps 3 to 7.
9 Afterward, rinse your mouth with water and spit it out. This may help prevent certain side effects in the mouth and throat. You can also brush your teeth.
10 Immediately after using the inhaler, always replace the mouthpiece cover so that it does not collect dust. Replace the cover by pushing it firmly until you hear a click as it snaps into place.
It is recommended that you practice in front of a mirror the first few times. If you see a kind of "mist" coming out of the top of the inhaler or from the corners of your mouth, you should start again.
It may be helpful for older children or people with little hand strength to hold the inhaler with both hands. Place your index fingers on the top of the inhaler and your thumbs on the bottom, below the mouthpiece. Your doctor, nurse, or pharmacist will show you how to do this.
Cleaning the inhaler
To avoid clogging the inhaler, you should clean it at least once a week.
To do this:
- Remove the mouthpiece cover.
- Do not remove the metal cartridge from the plastic casing.
- Clean the inside and outside of the mouthpiece and the plastic casing with a dry cloth or paper towel.
- Replace the mouthpiece cover.
Do not submerge the metal cartridge in water.
If you use more Fluticasona Cipla than you should
If you have used a higher dose than recommended, talk to your doctor as soon as possible.
It is important that you take the dose indicated on the prescription or as your doctor has told you. Do not increase or reduce the dose without consulting your doctor first.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 915 620 420, indicating the medicine and the amount ingested.
If you forget to use Fluticasona Cipla
- Take your next dose when it is due.
- Do not take a double dose to make up for the forgotten dose.
If you stop using Fluticasona Cipla
Do not stop your treatment, even if you feel better, unless your doctor tells you to. Patients who have been treated with high doses of corticosteroids for a long period of time should not stop taking the medicine without consulting their doctor first, as their asthma may worsen. Abruptly stopping treatment can also cause discomfort and may cause symptoms such as vomiting, numbness, nausea, headache, fatigue, loss of appetite, decreased blood glucose levels, and convulsions.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. If you experience any of the following serious side effects, stop using this medicine and consult your doctor immediately. You may need urgent medical treatment.
- Allergic reaction (may affect up to 1 in 100 people) or severe allergic reactions (may affect up to 1 in 10,000 people), signs include skin rash, redness, itching, or "hives-like" rash and swelling of the face, lips, mouth, tongue, or throat, which can cause difficulty swallowing or breathing, itchy rash, feeling of fainting and dizziness, and collapse, respectively.
- If your breathing or wheezing worsens just after using the inhaler.
Other side effects are:
Very common(may affect more than 1 in 10 people)
- thrush in the mouth and throat.
Common(affects less than 1 in 10 people)
- throat irritation and hoarseness
- bruising
- pneumonia (lung infection) in patients with COPD.
Tell your doctor if you have any of the following symptoms while inhaling Fluticasona Cipla, they could be symptoms of a lung infection:
- fever or chills
- increased production of mucus, change in the color of the mucus
- increased coughing or increased difficulty breathing
Very rare(affects less than 1 in 10,000 people)
- difficulty sleeping or feelings of worry, overexcitement, and irritability (these effects occur mainly in children),
- may increase blood sugar levels,
- using high doses of Fluticasona Cipla for long periods of time may cause: adrenal suppression, acute adrenal crisis, Cushing's syndrome, decreased bone density, eye problems (such as cataracts and glaucoma, which is high pressure in the eye), and growth retardation in children and adolescents (see Section 2 "Warnings and precautions").
To help prevent these symptoms, your doctor will make sure you are using the lowest dose of corticosteroids that controls your asthma.
Frequency not known(cannot be estimated from the available data)
- depression,
- feeling restless or nervous (these effects occur mainly in children),
- blurred vision.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Monitoring System https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Fluticasona Cipla
Keep this medicine out of the sight and reach of children.
Clean your inhaler weekly and if it becomes blocked, follow the instructions described in the "Cleaning the inhaler" section.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date is the last day of the month stated.
The cartridge contains a pressurized liquid. Do not expose to temperatures above 50°C. Do not pierce the cartridge. Do not refrigerate or freeze. Protect from very low temperatures and direct sunlight.
If the inhaler is very cold, remove the metal cartridge from the plastic casing and warm it in your hands for a few minutes before use. Never use other methods to heat it up.
The metal cartridge is pressurized. Do not pierce, break, or burn it, even if it appears to be empty.
Medicines should not be disposed of via wastewater or household waste. Return the packaging and any unused medicine to a pharmacy for proper disposal. By doing this, you will help protect the environment.
6. Container Content and Additional Information
Composition of Fluticasona Cipla
- The active ingredient is fluticasone propionate. Each measured dose contains 125 micrograms of fluticasone propionate, which is equivalent to an emitted dose of 110 micrograms of fluticasone propionate.
- This medication contains fluorinated greenhouse gases.
- Each inhaler contains 12.5 g of HFC-134a (norflurane) which corresponds to 0.018 tons of CO2 equivalent (global warming potential GWP = 1430).
Appearance of the Product and Container Content
- Fluticasona Cipla is a white suspension, contained in an aluminum alloy cartridge treated with fluorocarbon polymer, which is sealed with a metering valve, an applicator, and a protective cap.
- Package sizes: Single package - each package contains a bottle with 120 puffs.
Multipack - wrapped with 2 or 3 packages. Hospital package - wrapped with 10 packages. Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Cipla Europe NV
De Keyserlei 60C, Bus-1301,
2018, Antwerp,
Belgium
Manufacturer
S&D Pharma CZ, spol. s.r.o Theodor 28,
273 08 Pchery, (Pharmos a.s. facility),
Czech Republic
or
Cipla Europe NV
De Keyserlei 60C, Bus-1301,
2018, Antwerp,
Belgium
Local Representative
Cipla Europe NV branch in Spain,
C/ Guzmán el Bueno, 133 Edif Britannia-28003- Madrid
Spain
This medication is authorized in the Member States of the European Economic Area under the following names:
Sweden | Fluticasone Cipla 125 micrograms/dose inhalation spray, suspension |
Germany | Fluticason Cipla 125 micrograms/puff pressurized inhalation, suspension |
Italy | FLUTICASONE DOC 125 micrograms per actuation, pressurized inhalation suspension |
Norway | Flutikason Cipla 125 micrograms/dose inhalation aerosol, suspension |
Spain | Fluticasona Cipla 125 micrograms/inhalation suspension for inhalation in a pressurized container |
Date of the last revision of this prospectus: January 2025
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/

How much does FLUTICASONE CIPLA 125 micrograms/inhalation suspension for inhalation in pressurized container cost in Spain ( 2026)?
The average price of FLUTICASONE CIPLA 125 micrograms/inhalation suspension for inhalation in pressurized container in January, 2026 is around 15.74 EUR. Prices may vary depending on the region, pharmacy, and whether a prescription is required. Always check with a local pharmacy or online source for the most accurate information.
- Country of registration
- Average pharmacy price15.74 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FLUTICASONE CIPLA 125 micrograms/inhalation suspension for inhalation in pressurized containerDosage form: PULMONARY INHALATION, 250 mcg fluticasone propionateActive substance: fluticasoneManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: PULMONARY INHALATION, 50 mcg fluticasone propionateActive substance: fluticasoneManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: PULMONARY INHALATION, 100 µgActive substance: fluticasoneManufacturer: Glaxosmithkline S.A.Prescription required
Alternatives to FLUTICASONE CIPLA 125 micrograms/inhalation suspension for inhalation in pressurized container in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to FLUTICASONE CIPLA 125 micrograms/inhalation suspension for inhalation in pressurized container in Poland
Alternative to FLUTICASONE CIPLA 125 micrograms/inhalation suspension for inhalation in pressurized container in Ukraine
Online doctors for FLUTICASONE CIPLA 125 micrograms/inhalation suspension for inhalation in pressurized container
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for FLUTICASONE CIPLA 125 micrograms/inhalation suspension for inhalation in pressurized container – subject to medical assessment and local rules.