FLUTICASONE ALDO-UNION 1 mg/ml SUSPENSION FOR NEBULIZER INHALATION

How to use FLUTICASONE ALDO-UNION 1 mg/ml SUSPENSION FOR NEBULIZER INHALATION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is Fluticasone Aldo-Union 1 mg/ml and what is it used for
- What you need to know before you start using Fluticasone Aldo-Union 1 mg/ml
- How to use Fluticasone Aldo-Union 1 mg/ml
- Possible side effects
- Storage of Fluticasone Aldo-Union 1 mg/ml
- Package Contents and Additional Information
Introduction
Package Leaflet: Information for the User
Fluticasone Aldo-Union 1 mg/ml Suspension for Inhalation by Nebulizer
Fluticasone Propionate
Read this package leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this package leaflet, you may need to read it again. If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others, as it may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Fluticasone Aldo-Union 1 mg/ml and what is it used for
- What you need to know before you start using Fluticasone Aldo-Union 1 mg/ml
- How to use Fluticasone Aldo-Union 1 mg/ml
- Possible side effects
- Storage of Fluticasone Aldo-Union 1 mg/ml
- Contents of the pack and further information
1. What is Fluticasone Aldo-Union 1 mg/ml and what is it used for
This medicine contains the active ingredient fluticasone propionate, which belongs to a group of medicines called corticosteroids, and is used by inhalation as an anti-asthmatic (for the treatment of asthma, a disease characterized by reversible obstruction of the lower respiratory airways).
Fluticasone propionate reduces swelling and inflammation of the lungs (anti-inflammatory action).
This medicine is indicated for the prevention of chronic severe asthma in adults and adolescents over 16 years of age who require high-dose inhaled treatment or oral corticosteroids.
2. What you need to know before you start using Fluticasone Aldo-Union 1 mg/ml
Do not use Fluticasone Aldo-Union 1 mg/ml
- if you are allergic to fluticasone propionate or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before you start using Fluticasone Aldo-Union 1 mg/ml.
Especially, talk to your doctor if:
- You have active or latent tuberculosis (a contagious disease caused by a bacterium).
- You are diabetic.
- You suffer from a respiratory disease called Chronic Obstructive Pulmonary Disease (COPD).
- You are switching from treatment with oral steroids to treatment with fluticasone by inhalation. In this case, your doctor will treat you with special care, regularly monitor your adrenal function, and gradually stop the oral treatment after introducing the inhaled treatment. For this reason, it is recommended that you carry a badge indicating that you may need complementary treatment with corticosteroids during periods of stress.
The need to use medications for symptom control more frequently indicates a worsening of asthma control, and in such circumstances, you should consult your doctor to assess the possibility of changing your treatment.
Keep in mind that sudden worsening of asthma is potentially life-threatening.
It is essential that you use the dose prescribed by your doctor. Do not increase or decrease your dose without consulting your doctor. Do not stop treatment with fluticasone propionate abruptly.
When inhaled glucocorticoids (such as fluticasone propionate) are used, especially when prescribed at high doses for prolonged periods, systemic effects (affecting the whole body) may occur. The likelihood of these effects is lower than with oral glucocorticoid treatment.
Possible systemic effects include Cushing's syndrome (a disease characterized by an excess of glucocorticoids in the blood that produces obesity, lack of facial expression, red skin, extensive stretch marks, and dense hair, especially on the face), Cushingoid appearance (typical of people affected by Cushing's syndrome), adrenal suppression (reduction of adrenal gland function), growth retardation in children and adolescents, decreased bone mineral density, cataracts (opacity of the lens inside the eye), glaucoma (a severe eye disease), and, more rarely, a series of psychological or behavioral effects, including psychomotor hyperactivity, sleep disorders, anxiety, depression, or aggression (especially in children) (see section 4). Therefore, it is essential that the dose of inhaled corticosteroid is the lowest possible to maintain effective asthma control.
Contact your doctor if you experience blurred vision or other visual disturbances.
Replacing treatment with systemic corticosteroids with inhaled corticosteroids may trigger an allergic disease.
If you experience breathing difficulties (bronchospasm with increased dyspnea) after using Fluticasone Aldo-Union 1 mg/ml, stop taking the medicine and consult your doctor immediately.
This medicine is not suitable for use as the only medicine to treat symptoms of an acute bronchospasm attack and does not replace emergency treatment with oral or intravenous corticosteroids.
Children and adolescents
Fluticasone Aldo-Union 1 mg/ml should be used with caution in children.
Regular monitoring of the height of children receiving prolonged treatment with inhaled corticosteroids is recommended.
Very rare cases of acute adrenal insufficiency have been reported in children exposed to higher-than-recommended doses (approximately 1 mg per day) for prolonged periods (several months or years).
Using Fluticasone Aldo-Union 1 mg/ml with other medicines
Tell your doctor if you are taking or have recently taken any other medicines, including those obtained without a prescription.
In particular, tell your doctor or pharmacist if you are using any of the following medicines:
- ritonavir, a type of antiviral medicine known as a "protease inhibitor",
- ketoconazole, a medicine used to treat fungal infections (caused by fungi).
Some medicines may increase the effects of Fluticasone Aldo-Union 1 mg/ml, so your doctor will monitor you closely if you are taking these medicines (including some for HIV: ritonavir, cobicistat).
Using Fluticasone Aldo-Union 1 mg/ml with food and drinks
This medicine can be used at any time of day, with or without food.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
It is unlikely that this medicine will affect your ability to drive or use machines.
3. How to use Fluticasone Aldo-Union 1 mg/ml
Follow the instructions for administration of this medicine indicated by your doctor. If in doubt, consult your doctor or pharmacist again.
Using this medicine
- Do not inject or ingest the liquid contained in each plastic ampoule. Use Fluticasone Aldo-Union 1 mg/ml only through an aerosol generated by a nebulizer. Do not use this medicine with ultrasonic nebulizers.
- The liquid is introduced into the nebulizer, and it produces a mist that is inhaled through a mouthpiece or an inhalation mask.
- Do not allow the liquid or the mist produced by the nebulizer to come into contact with your eyes. You can wear normal or safety glasses to protect your eyes.
- Using a mouthpiece prevents the medicine from affecting the face, which can happen if a mask is used for extended periods. If you prefer to use a mask, or if you are using a mask for your child, you should protect the skin of the face with a cream or wash the face well after treatment. If you use a mask, nasal inhalation may occur.
- Use the nebulizer in a well-ventilated room, as the mist released into the air could also be inhaled by others.
A complete ampoule of this medicine (2 ml) provides a dose of 2 mg of fluticasone propionate. Each ampoule has a mark indicating half of its contents (1 ml).
Patients should be treated with an initial dose of fluticasone propionate for nebulization suitable for the severity of their disease.
Your doctor will prescribe the appropriate dosage regimen for this medicine after evaluating the severity of your asthma and your individual response. Your doctor may gradually reduce the dose if your respiratory function stabilizes.
Adults and adolescents over 16 years of age
For preventive treatment, the medicine should be taken regularly, even if you do not experience symptoms.
The recommended initial dose is usually 0.25 mg of fluticasone propionate 2 times a day. Depending on the individual response, the dose may be increased (0.5-2 mg of fluticasone propionate 2 times a day) until asthma control is achieved, or reduced to the minimum effective maintenance dose.
Children and adolescents under 16 years of age
The safety and efficacy of Fluticasone Aldo-Union 1 mg/ml suspension for inhalation by nebulizer in children and adolescents under 16 years of age have not been established. Current clinical data do not allow for adequate dosage recommendations in this patient population.
If you are using high doses of an inhaled steroid for a long time, you may occasionally need to use additional doses of steroids, for example, in stressful situations,such as a traffic accident or before surgery. Your doctor may decide to prescribe a steroid supplement during that period.
Patients who have been treated with high doses of steroids, such as Fluticasone Aldo-Union 1 mg/ml, for a long time, should not stop taking the medicine abruptly and without informing their doctor.Stopping treatment suddenly can cause discomfort and symptoms such as vomiting, drowsiness, nausea, headache, fatigue, loss of appetite, low blood sugar levels (hypoglycemia), and weight changes.
Instructions for use
- The ampoule is supplied wrapped. Do not open the ampoule until you need it.
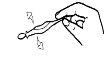
- Make sure the contents of the ampoule are well mixed before using it. Hold the ampoule horizontally, holding it by the lower end, gently tap the opposite end several times, and shake it. Repeat this procedure several times until the entire contents of the ampoule are completely mixed.
Tapping | Shaking |
|
|
To open the ampoule, twist the top firmly.
- Introduce the suspension into the nebulizer, following the product instructions for using the nebulizer.
Dilution of the suspension:
Do not dilute the contents of the suspension unless your doctor tells you to
- If your doctor has told you to dilute the solution, pour the contents of the ampoule into the nebulizer container
- Add the amount of physiological solution for injectable use that your doctor has indicated you should use.
- Put the lid on the nebulizer container and gently shake it to mix the contents.
After use:
- Use a new ampoule for each dose. Open a new one only when you are ready to use it. Discard any remaining liquid. Do not save it for reuse.
- Discard any remaining solution in the nebulizer container.
- Clean the nebulizer as recommended.
It is recommended to administer with a mouthpiece.
If an inhalation mask is used, wash your face well after treatment.
If you use more Fluticasone Aldo-Union 1 mg/ml than you should
It is essential that you take your dose as indicated by your doctor. Do not increase or decrease your dose without medical supervision.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately, or call the Toxicology Information Service, phone 915620420, indicating the medicine and the amount ingested.
Acute inhalation of Fluticasone Aldo-Union 1 mg/ml in doses higher than recommended may cause temporary suppression of adrenal function (for symptoms, see section 2). No emergency intervention is necessary if adrenal function returns to normal within a few days.
However, if higher-than-recommended doses are used for prolonged periods, some degree of adrenal insufficiency may occur. Your doctor may request tests to check the function of the adrenal gland.
If you forget to use Fluticasone Aldo-Union 1 mg/ml
- Take the next dose when it is due.
- Do not take a double dose to make up for forgotten doses.
If you stop treatment with Fluticasone Aldo-Union 1 mg/ml
- Do not stop treatmenteven if you feel better, unless your doctor tells you to. In that case, do not stop Fluticasone Aldo-Union 1 mg/ml abruptly.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop using the medicine and contact your doctor immediately if you experience any of the following symptoms after using Fluticasone Aldo-Union 1 mg/ml:
- allergic reactions characterized by:
- skin rashes (cutaneous hypersensitivity);
- swelling of the face, lips, mouth, tongue, or throat that can cause difficulty swallowing (angioedema)
- breathing difficulties (dyspnea and/or bronchospasm, paradoxical bronchospasm)
Other side effects are:
Very common(may affect more than 1 in 10 people)
- oral and throat candidiasis (painful yellowish-white plaques)
Common(may affect up to 1 in 10 people)
- bruises
- hoarseness (voice changes).
Mouth and throat problems can be reduced with some specific measures, such as brushing your teeth or rinsing your mouth with water immediately after inhaling the dose.
- pneumonia (lung infection) in patients with chronic obstructive pulmonary disease (COPD).
Very rare(may affect up to 1 in 10,000 people)
- esophageal candidiasis (thrush);
- Cushing's syndrome, a disorder characterized by an excess of steroid hormones in the bloodstream, which manifests with a rounded face;
- growth retardation;
- thinning of the bones (decreased bone mineral density);
- eye problems such as cataracts (lens opacity) and glaucoma (increased eye pressure);
- effects due to the adrenal gland (a small gland located next to the kidney);
- high blood sugar;
- anaphylactic reactions.
Frequency not known(cannot be estimated from available data):
Sleep disturbances, depression, or feelings of worry, anxiety, nervousness, excitability, or irritability (these effects are more likely to occur in children) and blurred vision.
Reporting side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Fluticasone Aldo-Union 1 mg/ml
Keep this medicine out of the sight and reach of children.
This medicine does not require special storage conditions. Keep the medicine in a vertical position, as shown on the packaging, inside the wrapping and box.
Do not use this medicine after the expiration date stated on the packaging after "EXP". The expiration date is the last day of the month indicated.
Once the aluminum wrapping is opened, the containers should be protected from light and used within 1 month. Do not store above 25°C.
Opened containers should be stored in the refrigerator (between 5°C ± 3°C) and used within 24 hours of opening. Do not freeze.
Medicines should not be disposed of via wastewater or household waste. Deposit the containers and medicines you no longer need at the SIGRE collection point in your pharmacy. If in doubt, ask your pharmacist how to dispose of containers and medicines you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Fluticasona Aldo-Unión 1 mg/ml
- The active ingredient is fluticasone propionate.
- The other components are polysorbate 20, sorbitan monolaurate, sodium dihydrogen phosphate dihydrate, sodium phosphate dibasic anhydrous, sodium chloride, water for injectable preparations.
Appearance of the Product and Package Contents
- Fluticasona Aldo-Unión 1 mg/ml is presented in strips of 5 ampoules in an aluminum wrapper. They are arranged in boxes containing 20 ampoules of 2 ml.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Laboratorio Aldo-Unión, S.L.
Calle Baronesa de Maldá 73
08950 Esplugues de Llobregat (Barcelona)
Spain
Manufacturer
Genetic S.p.A
Contrada Canfora
84084 Fisciano (SA)
Date of the Last Review of this Prospectus:August 2019
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es/
- Country of registration
- Average pharmacy price41.96 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FLUTICASONE ALDO-UNION 1 mg/ml SUSPENSION FOR NEBULIZER INHALATIONDosage form: PULMONARY INHALATION, 250 mcg fluticasone propionateActive substance: fluticasoneManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: PULMONARY INHALATION, 50 mcg fluticasone propionateActive substance: fluticasoneManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: PULMONARY INHALATION, 100 µgActive substance: fluticasoneManufacturer: Glaxosmithkline S.A.Prescription required
Online doctors for FLUTICASONE ALDO-UNION 1 mg/ml SUSPENSION FOR NEBULIZER INHALATION
Discuss questions about FLUTICASONE ALDO-UNION 1 mg/ml SUSPENSION FOR NEBULIZER INHALATION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions