

Buventol Easihaler

Ask a doctor about a prescription for Buventol Easihaler

How to use Buventol Easihaler
1. What is Buventol Easyhaler and what is it used for
Salbutamol belongs to a group of medicines called short-acting beta-2 receptor agonists, which cause the relaxation of smooth muscle in the airways. It causes the airways to open and thus helps to maintain airway patency. It relieves symptoms of respiratory disorders such as a feeling of tightness in the chest, wheezing, and coughing. The effect of salbutamol occurs after a few minutes and lasts for a short period (4 to 6 hours).
Buventol Easyhaler is indicated for adults and children over 6 years of age for the symptomatic treatment of asthma attacks and asthma exacerbations, as well as for the prevention of asthma attacks caused by physical exertion or expected contact with allergens that usually cause shortness of breath.
In adults, the medicine is also indicated for the symptomatic treatment of exacerbations of chronic obstructive pulmonary disease with reversible airway obstruction.
2. Important information before using Buventol Easyhaler
When not to use Buventol Easyhaler
- if the patient is allergic to salbutamol, milk proteins, or any of the other ingredients of this medicine (listed in section 6).
- in the case of threatened abortion or to prevent premature labor.
Warnings and precautions
Before starting treatment with Buventol Easyhaler, the patient should discuss it with their doctor or pharmacist if they have:
- high blood pressure,
- any heart or blood vessel disease,
- problems with blood sugar levels, including diabetes,
- hyperthyroidism,
- low potassium levels in the blood. The doctor may recommend monitoring potassium levels in the blood during treatment with Buventol Easyhaler.
If the patient experiences shortness of breath or chest pain, they should consult a doctor.
Buventol Easyhaler and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
In particular, the patient should tell their doctor if they are taking any of the following medicines:
- other medicines that dilate the airway muscles
- beta-adrenergic receptor blockers (e.g., propranolol) (used to treat high blood pressure or other heart diseases)
- theophylline or aminophylline (used to treat asthma or chronic bronchitis)
- medicines used to treat depression
- diuretics
- digitalis glycosides (medicines used to treat heart diseases)
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
The medicine should not be used in pregnant or breastfeeding women, unless the doctor recommends otherwise.
Driving and using machines
There is no data on the effect of the medicine on the ability to drive and use machines.
Buventol Easyhaler contains lactose
The medicine contains a small amount of lactose (milk sugar), which should not cause problems for patients with lactose intolerance. If the patient has previously been diagnosed with intolerance to some sugars, they should contact their doctor before taking the medicine.
3. How to use Buventol Easyhaler
This medicine should always be used as directed by the doctor or pharmacist. If in doubt, the patient should consult their doctor or pharmacist.
The active substance of the medicine, salbutamol, is in the Easyhaler inhaler. The medicine is a dry powder intended for inhalation through the mouthpiece of the inhaler.
Buventol Easyhaler should be used as needed, and not regularly.
Recommended dose:
Adults:
- For the treatment of acute bronchospasm, 100 µg (micrograms) to 200 µg of salbutamol should be used.
- For the prevention of asthma attacks caused by physical exertion or expected contact with allergens, 100 µg or 200 µg of salbutamol should be administered 15-30 minutes before physical exertion or expected contact with the allergen.
Children over 6 years of age:
- For the treatment of acute bronchospasm, the recommended initial dose is 100 µg of salbutamol. If necessary, the dose can be increased to 200 µg of salbutamol.
- For the prevention of asthma attacks caused by physical exertion or allergens, 100 µg or 200 µg of salbutamol should be administered 15-30 minutes before physical exertion or expected contact with the allergen.
Buventol Easyhaler is not indicated for use in children under 6 years of age.
For adults and children, the maximum daily dose (within 24 hours) is 800 μg (8 inhalations of Buventol Easyhaler, 100 micrograms/dose, or 4 inhalations of Buventol Easyhaler, 200 micrograms/dose).
IT IS VERY IMPORTANT NOT TO TAKE MORE OF THIS MEDICINE THAN RECOMMENDED BY THE DOCTOR.
Important
If the patient's asthma is active (e.g., frequent symptoms or exacerbations, such as shortness of breath that interferes with speaking, eating, or sleeping, coughing, wheezing, chest tightness, or limited physical performance), they should immediately tell their doctor, who may initiate treatment or increase the dose of a medicine that helps control asthma symptoms, such as an inhaled corticosteroid.
If the patient thinks the medicine is not working as well as usual, they should tell their doctor as soon as possible (e.g., the patient needs higher doses to relieve breathing problems or asthma symptoms do not improve for at least 3 hours after using the inhaler), as asthma may be worsening and another medicine may be needed.
If the patient uses Buventol Easyhaler more frequently than twice a week to relieve asthma symptoms, not including preventive use before physical exertion, it means that asthma is not well-controlled and may increase the risk of severe asthma attacks (asthma exacerbations), which can cause serious complications and be life-threatening. The patient should contact their doctor as soon as possible to verify their asthma treatment.
If the patient uses a medicine with anti-inflammatory effects in the lungs, such as an "inhaled corticosteroid", every day, it is essential to continue using it regularly, even if the patient feels better.
If the patient thinks the effect of Buventol Easyhaler is too strong or too weak, they should consult their doctor.
Instructions for using the inhaler are at the end of the leaflet.
Using a higher dose of Buventol Easyhaler than recommended
If a higher dose of the medicine is taken than recommended, it may cause an increased heart rate and a feeling of trembling. These symptoms should resolve on their own within a few hours, but the patient should inform their doctor or pharmacist. Additionally, it may cause a decrease in potassium levels and an increase in glucose in the blood, as well as symptoms suggesting the development of lactic acidosis (increased lactate levels in the blood), such as abdominal pain, increased ventilation (rapid breathing), shortness of breath despite decreased wheezing, cold hands and feet, irregular heartbeat, and increased thirst.
Missing a dose of Buventol Easyhaler
If a dose of Buventol Easyhaler is missed, the patient should take the next dose at the usual time or take the medicine before symptoms of shortness of breath occur. The doctor may recommend taking the medicine regularly every day or only when the patient experiences shortness of breath or shallow breathing. If the patient has any further doubts about using this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If the patient experiences any of the following symptoms, they should stop using Buventol Easyhaler and contact their doctor immediately:
- Uncommon (may affect up to 1 in 100 people): itching, rash, redness of the skin, swelling of the eyelids, lips, face, or throat, wheezing, low blood pressure, or collapse. These may be symptoms of a severe allergic reaction(anaphylaxis or angioedema).
- Rare (may affect up to 1 in 1,000 people): worsening of wheezing and shortness of breath (paradoxical bronchospasm) immediately after using the inhaler.
Other possible side effects:
Common(may affect up to 1 in 10 people)
- headache
- rapid heartbeat, palpitations (feeling of faster or stronger than usual heartbeat)
- peripheral vasodilation
- tremors
Uncommon(may affect up to 1 in 100 people)
- dryness of the oral mucosa, irritation of the oral mucosa and throat - rinsing the mouth and throat after inhalation can prevent irritation
Rare(may affect up to 1 in 1,000 people)
- decrease in potassium levels in the blood
- restlessness
- dizziness
- insomnia
- hyperactivity in children
- cough
- muscle cramps
- muscle pain
Very rare(may affect up to 1 in 10,000 people)
- feeling of uneven heartbeat
Frequency not known(cannot be estimated from the available data)
- myocardial ischemia (symptom may be, for example, chest pain)
Reporting side effects
If side effects occur, including any side effects not listed in the leaflet, the patient should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products,
Al. Jerozolimskie 181 C, 02-222 Warsaw, Tel.: +48 22 49 21 301,
Fax: + 48 22 49 21 309, Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the medicine.
5. How to store Buventol Easyhaler
Keep out of sight and reach of children.
Store in a temperature below 25 ° C.
Before first use, the medicine should be stored in its original packaging.
After opening the aluminum foil packaging, the medicine should be protected from moisture. It is recommended to store the inhaler in a protective container.
In case of moisture, the inhaler should be replaced with a new one.
The inhaler should be replaced 6 months after opening the packaging. To remember the opening date, it should be written in the box:
Do not use Buventol Easyhaler after the expiry date stated on the packaging after:
Expiry date (EXP). The expiry date refers to the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Buventol Easyhaler contains
- The active substance of the medicine is salbutamol in the form of salbutamol sulfate. Buventol Easyhaler, 100 micrograms/dose:One metered dose contains 100 micrograms (µg) of salbutamol in the form of salbutamol sulfate 120.5 µg. One delivered dose contains 90 micrograms (µg) of salbutamol in the form of salbutamol sulfate 108.5 µg.
Buventol Easyhaler, 200 micrograms/dose:
One metered dose contains 200 micrograms (µg) of salbutamol in the form of salbutamol sulfate 241 µg.
One delivered dose contains 180 micrograms (µg) of salbutamol in the form of salbutamol sulfate 217 µg.
- The other ingredient of the medicine is lactose monohydrate (contains milk proteins).
What Buventol Easyhaler looks like and contents of the pack
White or almost white powder.
1 Easyhaler inhaler contains 200 doses.
The plastic Easyhaler inhaler is equipped with a mouthpiece and dose counter, packaged in a laminated aluminum foil bag (PET/Al/PE) and placed in a cardboard box.
Pack size:
- 200 doses in an inhaler
- 200 doses in an inhaler with a protective container
Marketing authorization holder
Orion Corporation
Orionintie 1
FI-02200 Espoo
Finland
Manufacturer
Orion Corporation
Orionintie 1
FI-02200 Espoo
Finland
To obtain more detailed information, the patient should contact the representative of the marketing authorization holder:
Orion Pharma Poland Sp. z o.o.
[email protected]
Date of last revision of the leaflet:
Detailed and up-to-date information about this product, regarding the use of this medicine, is available by scanning the QR code on the patient leaflet, on the outer packaging of the medicine, and on the inhaler label. The same information is also available on the website: www.oeh.fi/spl
QR code for the website: www.oeh.fi/spl
How to use the Easyhaler inhaler
Information about the Easyhaler inhaler
The Buventol Easyhaler inhaler may differ from inhalers used by the patient in the past.
Therefore, it is very important to use it correctly, as incorrect use may cause the patient not to receive the correct amount of medicine. This may lead to worsening of the patient's condition or inadequate treatment of asthma or COPD (chronic obstructive pulmonary disease).
The doctor, nurse, or pharmacist will show the patient how to use the inhaler correctly.
The patient should make sure they understand how to use the inhaler correctly. If in doubt, they should contact their doctor, nurse, or pharmacist. As with all inhalers, caregivers should ensure that children prescribed Buventol Easyhaler use the correct inhalation technique, as described below. The patient can also use the video instructions at: www.oeh.fi/spl
Using the Easyhaler inhaler for the first time
| The Easyhaler inhaler is supplied in a laminated bag. The bag should not be opened until the patient is ready to start treatment, as it helps to keep the powder in the inhaler dry. If the patient is ready to start treatment, they should open the packaging and record the opening date, e.g., in a calendar. The Buventol Easyhaler inhaler can be used for 6 months after removal from the foil bag. | 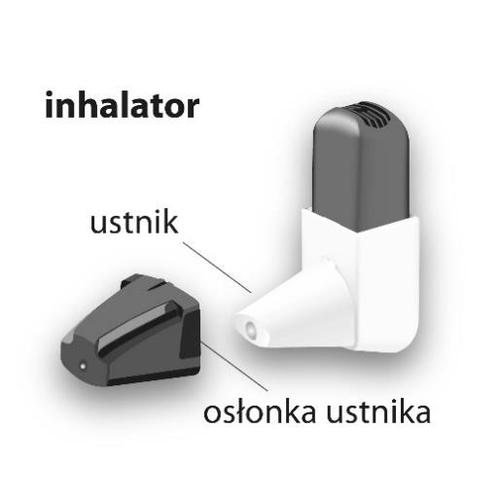 |
HOW TO USE THE INHALER
Step 1: SHAKING
| SHAKE 3 TO 5 TIMES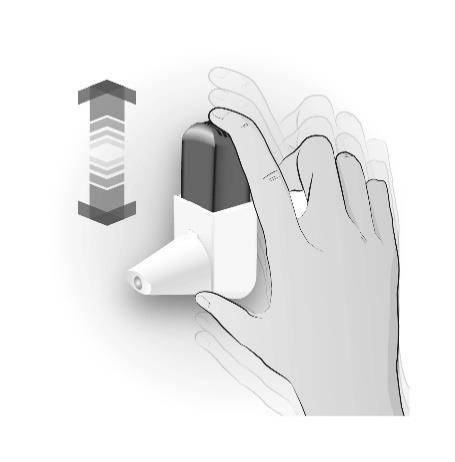 | Important information to remember
|
Step 2: CLICKING
| CLICK 1 TIME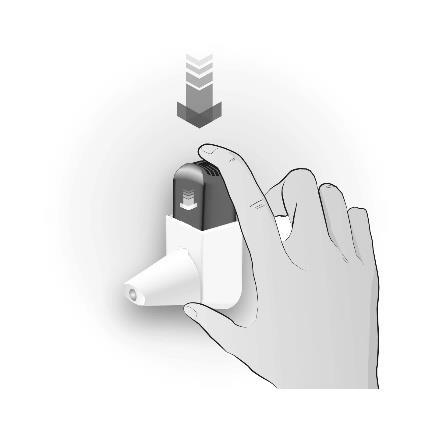 | Important information to remember
|
| before inhaling. |
If an additional inhalation is necessary, the steps described in points 1-3 "Shaking, Clicking, Inhaling" should be repeated.
After using the inhaler:
- Replace the mouthpiece cover to prevent accidental activation of the inhaler.
Step 3: INHALATION
| INHALATION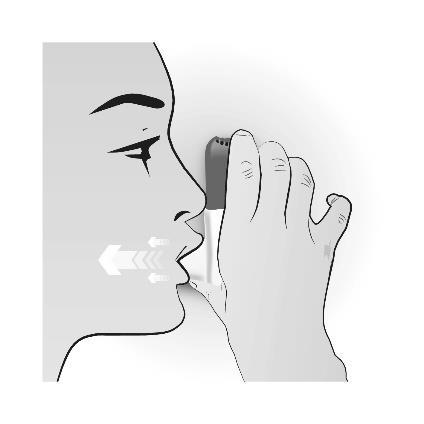 | Important information to remember
|
How to remove powder from the mouthpiece In case of accidental clicking of the inhaler or multiple clicking of the inhaler, or exhaling into the inhaler, the powder should be removed from the mouthpiece:
|  |
Cleaning the Easyhaler inhaler
The inhaler should be kept dry and clean. If necessary, the mouthpiece of the inhaler can be cleaned with a dry cloth or tissue. Do not use water. The powder in the Easyhaler inhaler is sensitive to moisture.
| Using the Easyhaler inhaler in a protective container The inhaler can be used in a protective container, which will increase the product's durability. Before placing the inhaler in the protective container, make sure the mouthpiece cover is covering the mouthpiece, preventing accidental activation of the inhaler. The inhaler can be used without removing it from the protective container. |
| Use according to the above instructions, 1. Shaking, 2. Clicking, 3. Inhaling. Remember to: |
|  |
| Replacing the Easyhaler inhaler with a new one The inhaler is equipped with a dose counter, which indicates the number of doses remaining. The counter rotates every five activations. When the dose counter changes color to red, it means there are 20 doses left. If the patient does not have a new Easyhaler inhaler, they should contact their doctor to obtain a new prescription. When the counter shows 0, the Easyhaler inhaler should be replaced. If the patient uses a protective container, they should keep it and place the new inhaler in it. | 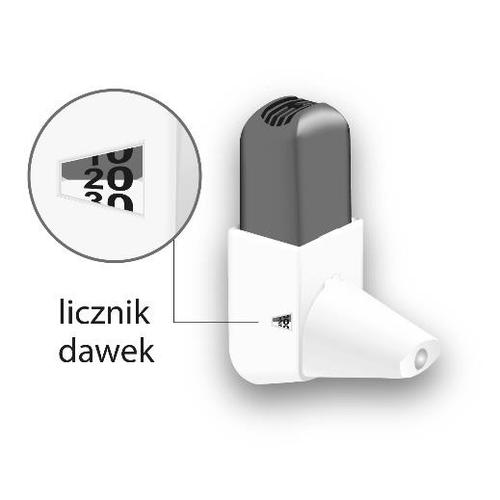 |
To remember
- 1. Shaking, 2. Clicking, 3. Inhaling.
- Do not allow the inhaler to get wet, keep it away from moisture.
If the patient has any further questions about using this medicine, they should contact their doctor or pharmacist.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterOrion Corporation
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Buventol EasihalerDosage form: Aerosol, 100 mcg/dose inh.Active substance: salbutamolManufacturer: Laboratorio Aldo-Union S.A.Prescription requiredDosage form: Powder, 100 mcg/doseActive substance: salbutamolManufacturer: Orion CorporationPrescription requiredDosage form: Aerosol, 100 mcg/doseActive substance: salbutamolManufacturer: Aeropharm GmbHPrescription required
Alternatives to Buventol Easihaler in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Buventol Easihaler in Spain
Alternative to Buventol Easihaler in Ukraine
Online doctors for Buventol Easihaler
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Buventol Easihaler – subject to medical assessment and local rules.














