
VENTOALDO 100 mcg/DOSE PRESSURIZED INHALATION SUSPENSION

How to use VENTOALDO 100 mcg/DOSE PRESSURIZED INHALATION SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is Ventoaldo 100 micrograms/dose and what is it used for
- What you need to know before starting to use Ventoaldo 100 micrograms/dose
- How to use Ventoaldo 100 micrograms/dose
- Possible side effects
- Storage of Ventoaldo 100 micrograms/dose
- Contents of the container and additional information
Introduction
Leaflet: information for the user
Ventoaldo 100 micrograms/dose inhalation suspension in pressurized container
salbutamol
Read the entire leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this leaflet, as you may need to read it again. If you have any doubts, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet
- What is Ventoaldo 100 micrograms/dose and what is it used for
- What you need to know before starting to use Ventoaldo 100 micrograms/dose
- How to use Ventoaldo 100 micrograms/dose
- Possible side effects
- Storage of Ventoaldo 100 micrograms/dose
- Contents of the container and additional information
1. What is Ventoaldo 100 micrograms/dose and what is it used for
Salbutamol belongs to the group of medications called bronchodilators that act by relaxing the muscles of the walls of the small airways in the lungs. It facilitates breathing and relieves coughing.
- Symptomatic treatment of bronchospasm (closure of airways in the lungs) in bronchial asthma and in other processes associated with reversible obstruction of the airways. These are a group of lung diseases that cause inflammation of the airways, resulting in a blockage of airflow in the lungs.
- Prevention of bronchospasm (closure of airways in the lungs) induced by physical exercise or before exposure to a known and unavoidable allergenic stimulus (substance capable of producing an allergic reaction).
2. What you need to know before starting to use Ventoaldo 100 micrograms/dose
Do not use Ventoaldo 100 micrograms/dose:
- If you are allergic to salbutamol or any of the other components of this medication (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use this medication:
- If you have a history of heart disease, irregular heartbeat, or angina pectoris.
- If you suffer from thyrotoxicosis, diabetes mellitus, severe cardiovascular disorders, or hypertension (high blood pressure).
- If your condition worsens during treatment, you should consult your doctor, as you may need another treatment.
Other medications and Ventoaldo 100 micrograms/dose
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
It is important that you inform your doctor if you are taking any of the following medications:
- Non-cardioselective beta-blockers, as they are contraindicated in asthmatic patients. Propranolol and similar medications counteract the effects of salbutamol.
- During treatment with salbutamol, it is preferable not to administer imipramine, chlorpromazine, or chlordiazepoxide.
- Medications that decrease serum potassium, as the effects may be additive if administered together.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
The use of salbutamol is not recommended during pregnancy, and it should only be administered when the expected benefit to the mother is greater than any possible risk to the fetus.
In the case of administration to a breastfeeding mother, it is recommended to substitute breastfeeding.
Driving and using machines:
Although no effects on the ability to drive and use machines are expected, you should take into account the possibility of muscle cramps and tremors.
Use in athletes
This medication contains salbutamol, which may produce a positive result in doping tests.
Ventoaldo 100 micrograms/dose contains ethanol
This medication contains 3 mg of alcohol (ethanol) per puff. The amount per puff of this medication is equivalent to less than 1 ml of beer or 1 ml of wine. The small amount of alcohol in this medication does not produce any noticeable effect.
3. How to use Ventoaldo 100 micrograms/dose
Follow the administration instructions of this medication indicated by your doctor or pharmacist exactly. If in doubt, consult your doctor or pharmacist again.
Remember to take your medication.
This medication is administered by inhalation.
Ventoaldo 100 micrograms/dose should be used as needed and not regularly.
If your asthma is active (e.g., you have symptoms or frequent crises, such as difficulty breathing that makes it hard to speak, eat, or sleep, coughing, wheezing, chest tightness, or limited physical capacity), you should inform your doctor immediately, who may start administering another medication or increase the treatment dose, such as an inhaled corticosteroid, to control your asthma.
Tell your doctor as soon as possible if your medication seems to be not working as well as usual (e.g., if you need higher doses to relieve your respiratory problems or if your inhaler does not provide relief for at least 3 hours), as your asthma may be worsening, and you may need a different medication.
If you use Ventoaldo 100 micrograms/dose more than twice a week to treat your asthmatic symptoms, excluding preventive use before exercise, this indicates poorly controlled asthma and may increase the risk of severe asthma attacks (worsening of asthma) that can have serious complications and can be life-threatening. You should contact your doctor as soon as possible to review your asthma treatment.
If you use a medication against inflammation of your lungs daily, e.g., an "inhaled corticosteroid," it is essential to continue using it regularly, even if you feel better.
The recommended dose is:
Adults
To relieve acute bronchospasm and treat intermittent asthma episodes, one inhalation can be administered as a single dose, which can be increased to two inhalations if necessary. If the response is inadequate, higher doses than two inhalations can be used. The maximum recommended dose is two inhalations, three or four times a day.
To prevent exercise-induced bronchospasm, one or two inhalations should be administered 15 minutes before exercise.
One or two inhalations can be administered before a planned contact with allergens.
Use in advanced age:
The same recommendations as for adults.
Use in children and adolescents
The recommended dose for relieving acute bronchospasm in the treatment of episodic asthma or for preventing exercise-induced asthma is one inhalation. If the response is inadequate, higher doses than one inhalation can be administered.
It is very important to follow the instructions indicated by the doctor.
Instructions for the correct administration of the preparation:
- Remove the cap (fig. 1). If it is a new inhaler or has not been used for several days, shake the aerosol (fig. 2) and perform a puff to ensure the proper functioning of the inhaler. If the inhaler is used regularly, proceed to the following instructions:
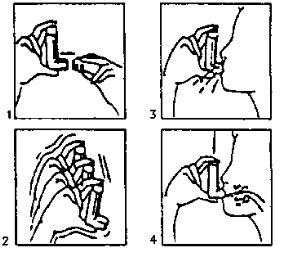 Shake the inhaler (fig. 2).
Shake the inhaler (fig. 2).- Eliminate as much air as possible from your lungs.
- Adapt the aerosol to your mouth according to the position indicated in the drawing (fig. 3).
- Make a deep inhalation.
You should press, according to the arrows in the drawing (fig. 4), the device while making this inhalation.
- Remove the aerosol from your mouth and try to hold your breath for a few seconds.
- You should periodically wash the oral adapter of the aerosol. To do this, remove the adapter from the aerosol and clean it with a cloth or paper towel. Store it with the cap on and protect it from dust and dirt.
The inhaler has a dose indicator that can be seen through a small hole or window on the actuator and indicates how many applications are left. In a new inhaler, the number "200" can be read through the window on the actuator. This number corresponds to the dose left in the inhaler. As the inhaler is used, the dose indicator rotates in a decreasing manner every 5-7 puffs until it reaches 0.
When there are approximately 40 doses left, the indicator changes from green to red (see figure 5) to remind the patient to consult their doctor if they need to continue treatment or if they need a new prescription. Discard the inhaler once the indicator reaches "0".

(figure 5)
If you use more Ventoaldo 100 micrograms/dose than you should
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service. Phone 91 562 04 20, indicating the medication and the amount ingested.
In case of taking a higher dose than recommended, peripheral vasodilation, increased heart rate, and muscle tremors may occur. This symptomatology disappears spontaneously. To counteract the effects of adrenergic beta-stimulation, cardioselective beta-blockers (practolol) can be used. Other adrenergic beta-blockers are not recommended, as they may cause bronchoconstriction in asthmatics. In case of acute intoxication, if ventricular arrhythmia occurs, it is recommended to administer a slow intravenous infusion of potassium chloride, 40 mEq in 500 ml of 5% dextrose solution.
If you forget to use Ventoaldo 100 micrograms/dose
Do not take a double dose to make up for forgotten doses. Inhale the next dose when it is due or sooner if you notice shortness of breath or "wheezing".
If you have any other doubts about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone experiences them.
Side effects are dose-dependent and are due to the mechanism of action of beta-2 agonists.
Although the exact frequency is not known, some people may occasionally experience chest pain (due to heart problems such as angina pectoris). Inform your doctor if you develop these symptoms while being treated with salbutamol, but do not stop taking this medication unless your doctor indicates it.
In very rare cases, allergic reactions have been reported, including angioedema (inflammation of the deep layers of the skin that manifests as large hives around the eyes and lips, which can also affect the hands, feet, and throat) and urticaria, bronchospasm, hypotension (low blood pressure), and fainting.
Disorders of the circulatory and lymphatic systems: potentially severe hypokalemia may occur as a consequence of systemic treatment with beta-2 agonists.
Psychiatric disorders: nervousness, feeling of tension. As with other beta-2 agonists, hyperactivity has been rarely reported in children.
Nervous system disorders: mild tremor, headache, dizziness.
Cardiovascular disorders: tachycardia, angioedema, hypotension. Cases of cardiac arrhythmias (including atrial fibrillation, supraventricular tachycardia, and extrasystole) have been reported in association with beta-2 agonists, usually in susceptible patients.
Respiratory, thoracic, and mediastinal disorders: as with other inhalation therapies, the possibility of paradoxical bronchospasm should be considered, with an immediate increase in wheezing after administration.
Gastrointestinal disorders: nausea.
Skin and subcutaneous tissue disorders: urticaria.
Musculoskeletal and connective tissue disorders: transient muscle cramps have been rarely reported.
General disorders and administration site conditions: cases of oral and pharyngeal irritation may occur.
If you observe any other adverse reaction not described above, consult your doctor or pharmacist.
Reporting of side effects:
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect that does not appear in this leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use: https://www.notificaram.es By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Ventoaldo 100 micrograms/dose
Keep this medication out of the sight and reach of children.
Do not store at a temperature above 30°C. Store in the original packaging to protect it from light. Do not freeze.
The container contains a pressurized liquid. Do not expose to temperatures above 50°C. Do not puncture the container, even if it appears to be empty.
Do not use this medication after the expiration date shown on the label and carton after EXP. The expiration date is the last day of the month indicated.
Medications should not be disposed of through wastewater or household waste. Deposit the containers and medications you no longer need at the SIGRE collection point in the pharmacy. If in doubt, ask your pharmacist how to dispose of the containers and medications you no longer need. This will help protect the environment.
6. Contents of the container and additional information
Composition of Ventoaldo 100 micrograms/dose
-The active ingredient is salbutamol base (equivalent to 120 micrograms of salbutamol sulfate)
-The other components (excipients) are oleic acid, ethanol, and Norflurane.
Appearance of the product and contents of the container
Ventoaldo 100 micrograms/dose is presented as a suspension in a pressurized container with a dose indicator; each box contains a 10 ml container that allows for 200 applications.
Marketing authorization holder and manufacturer
Laboratory Aldo-Unión, S.L.
Baronesa de Maldá, 73
08950 Esplugues de Llobregat (Barcelona)
Spain
Date of the last revision of this leaflet:
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VENTOALDO 100 mcg/DOSE PRESSURIZED INHALATION SUSPENSIONDosage form: PULMONARY INHALATION, 0.12% w/v SALBUTAMOL SULFATEActive substance: salbutamolManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: PULMONARY INHALATION, 0.24% w/v SALBUTAMOL SULFATEActive substance: salbutamolManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: PULMONARY INHALATION, 100 micrograms/applicationActive substance: salbutamolManufacturer: Laboratorio Aldo Union S.L.Prescription required
Online doctors for VENTOALDO 100 mcg/DOSE PRESSURIZED INHALATION SUSPENSION
Discuss questions about VENTOALDO 100 mcg/DOSE PRESSURIZED INHALATION SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















