
Aspulmo

Ask a doctor about a prescription for Aspulmo

How to use Aspulmo
Leaflet accompanying the packaging: patient information
Aspulmo, 100 micrograms per inhalation dose, inhalation aerosol, suspension
Salbutamol
Please read this leaflet carefully before using the medicine, as it contains important information for the patient.
- Please keep this leaflet, so you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Aspulmo and what is it used for
- 2. Important information before using Aspulmo
- 3. How to use Aspulmo
- 4. Possible side effects
- 5. How to store Aspulmo
- 6. Contents of the pack and other information
1. What is Aspulmo and what is it used for
Salbutamol, the active substance of Aspulmo, belongs to a group of bronchodilators.
It causes short-term (4-6 hours), rapid (within 5 minutes) bronchodilation
in case of reversible bronchial obstruction.
Aspulmo inhalation aerosol is recommended:
- for the treatment of bronchospasm or reversible bronchial obstruction;
- prophylactically before physical exertion in patients with exercise-induced asthma, as well as before predicted contact with allergens that usually cause bronchospasm. Aspulmo is particularly recommended for the treatment of mild, moderate or severe asthma, provided that its use does not delay the initiation of inhaled glucocorticosteroid therapy.
2. Important information before using Aspulmo
When not to use Aspulmo:
- if the patient is hypersensitive to salbutamol or any of the other ingredients of this medicine (listed in section 6); you should carefully read the information about hypersensitivity reactions contained in section 4 of this leaflet;
- in the case of threatened abortion;
- for the prevention of premature labor. In the case of premature labor or threatened abortion, the doctor may decide to administer salbutamol not by inhalation, but intravenously or in tablet form.
Warnings and precautions
To ensure optimal delivery of the inhaled medicine to the lungs, the doctor, nurse or pharmacist
will check the patient's inhalation technique.
Patients may feel a different taste compared to the previously used
inhalation medicine.
If the response to the previously used dose of the medicine is weaker or if it is necessary to take
more inhalations than before, you should immediatelycontact your doctor.
Aspulmo should be used with caution in patients with the following conditions:
- hyperthyroidism;
- heart failure;
- hypertension;
- aneurysm;
- decreased glucose tolerance;
- diabetes;
- phaeochromocytoma;
- myocardial ischemia;
- tachyarrhythmia;
- hypertrophic cardiomyopathy.
Aspulmo should be used with caution in patients taking glucocorticosteroids concomitantly.
Patients with severe heart disease who are using salbutamol should consult their doctor if they experience chest pain or other symptoms indicating worsening heart disease. Particular attention should be paid to symptoms such as shortness of breath and chest pain, which may result from cardiac or respiratory disorders.
If symptoms worsen during treatment, medical advice should be sought, as it may be necessary to change the treatment.
Aspulmo contains ingredients that may give a positive result in anti-doping tests.
Aspulmo and other medicines
Please tell your doctor or pharmacist about all the medicines you are taking now or have taken recently, as well as any medicines you plan to take.
Before starting treatment with Aspulmo, you should inform your doctor about the use of any of the following medicines:
- medicines that cause bronchospasm. These are beta-adrenergic receptor blockers (e.g. propranolol, atenolol) used to treat high blood pressure or angina pectoris. Aspulmo should not be used with these medicines;
- cardiac glycosides (e.g. digoxin) used in heart diseases. Cardiac glycosides increase the strength of heart contractions and at the same time make them less frequent;
- medicines that reduce potassium levels in the blood, as salbutamol may reduce potassium levels in the blood. These include:
- diuretics used to treat high blood pressure, heart failure, kidney failure, edema, or poisoning;
- xanthine derivatives (e.g. theophylline, aminophylline) - medicines used to treat asthma or other chronic obstructive pulmonary diseases;
- steroids (medicines used to treat asthma and other inflammatory diseases);
- medicines that may increase the risk of cardiovascular side effects in patients using Aspulmo. These include monoamine oxidase inhibitors (e.g. isocarboxazid, iproniazid, tranylcypromine, clorgyline, selegiline, moclobemide) or tricyclic antidepressants (e.g. imipramine, desipramine, clomipramine, amitriptyline).
Salbutamol should not be taken concomitantly with other inhaled bronchodilator medicines.
After the administration of certain general anesthetics (e.g. halothane, methoxyflurane or enflurane) to patients treated with salbutamol, an increased risk of severe cardiac arrhythmias and hypotension can be expected. If the administration of these medicines is planned, salbutamol should not be used for at least 6 hours before the start of anesthesia.
Pregnancy and breastfeeding
Before using any medicine, you should consult a doctor or pharmacist.
Use during pregnancy
The medicine can be used during pregnancy only if the benefits to the mother significantly outweigh
the risks to the fetus.
Use during breastfeeding
In cases where the medicine needs to be administered to breastfeeding women, it is recommended to stop breastfeeding. Salbutamol may pass into breast milk, and it is not known whether it has a negative effect on the health of newborns, so the use of salbutamol in breastfeeding women should be limited to cases where the expected benefit to the mother is greater than the potential risk to the child.
Driving and using machines
The medicine may affect the ability to drive and use machines.
If side effects such as muscle spasms or tremors occur, which may impair the ability to drive and use machines, caution should be exercised when performing these activities.
Aspulmo contains ethanol
This medicine contains 3 mg of alcohol (ethanol) per inhalation dose. The amount of alcohol in a dose of this medicine is equivalent to less than 1 ml of beer or 1 ml of wine.
A small amount of alcohol in this medicine will not have noticeable effects.
3. How to use Aspulmo
Aspulmo should always be used as directed by the doctor. In case of doubts, you should consult a doctor or pharmacist.
Aspulmo should be used as needed, and not regularly.
If the patient's asthma is active (e.g. frequent symptoms or exacerbations, such as shortness of breath that interferes with speaking, eating, or sleeping, coughing, wheezing, chest tightness, or limited physical fitness), they should immediately tell their doctor, who may start treatment or increase the dose of a medicine that helps control asthma symptoms, such as an inhaled corticosteroid.
If the patient thinks that the medicine is not working as well as usual, they should tell their doctor as soon as possible (e.g. the patient needs higher doses to relieve breathing problems or asthma symptoms do not improve for at least 3 hours after using the inhaler),
because asthma may worsen and another medicine may be needed.
If the patient uses Aspulmo more often than twice a week to relieve asthma symptoms, excluding prophylactic use before physical exertion, it means that asthma is poorly controlled and the risk of severe asthma attacks (asthma exacerbations) may increase, which can cause serious complications and be life-threatening. You should contact your doctor as soon as possible to verify the asthma treatment.
Adults and the elderly:
To relieve bronchospasm, one to two inhalations (100 micrograms to 200 micrograms of salbutamol) should be performed. If no improvement is observed in the patient, more than two inhalations can be performed.
The maximum recommended dose is two inhalations three times a day.
If the previously effective dose of inhaled salbutamol does not bring improvement within three hours, you should consult a doctor.
Prophylactically, before exertion or predicted contact with an allergen, two inhalations (2 x 100 micrograms of salbutamol) should be performed 10-15 minutes before exertion or predicted contact with the allergen.
One press of the inhaler releases one dose of the medicine.
Children:
Both for the treatment of acute bronchospasm and for exercise-induced asthma, one inhalation (100 micrograms of salbutamol) is recommended.
If necessary, the dose can be increased to 200 micrograms (2 x 100 micrograms of salbutamol).
It is not recommended to take more than four inhalations per day.
If you feel that the effect of Aspulmo is too strong or too weak, you should consult a doctor.
Do not stop using salbutamol without consulting your doctor.
Instructions for using the medicine
To ensure that the inhaler is working, you should remove the protective cap, then shake the inhaler vigorously and release a dose of the medicine into the air.
Instructions for using the inhaler:
- 1. Remove the protective cap from the mouthpiece.


- 2. Shake the inhaler vigorously.
- 3. Perform a possible deep exhalation.
- 4. Insert the mouthpiece of the inhaler into your mouth and seal it with your lips, as shown in the picture.


- 5. When inhaling through your mouth, press the inhaler, as shown in the picture.
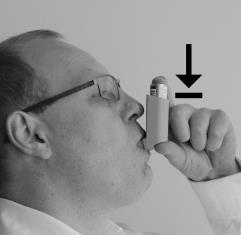
- 6. Remove the inhaler from your mouth and try to hold your breath for a few seconds or as long as possible. After this time, perform a calm exhalation.
- 7. Rinsing the mouth with water and spitting it out after inhalation prevents hoarseness and thrush.
- 8. After inhalation, you should always put the protective cap on the mouthpiece to prevent dust from entering it. Put the cap on by pressing it into the correct position until you hear a "click".
- 9. Store it with the cap to protect it from dust and dirt.
Contents of the inhaler:
You should shake the inhaler to check the amount of medicine left. Aspulmo should not be used if, when shaken, no liquid is found in the inhaler.
Cleaning the inhaler
To prevent the inhaler from clogging, it should be cleaned at least once a week.
To clean the inhaler:
- 1. Remove the protective cap from the mouthpiece.
- 2. Do not remove the inhaler from the plastic casing during cleaning or in other situations.

- 3. Clean the mouthpiece inside and outside and the plastic casing outside with a dry cloth or tissue.
- 4. Put the protective cap on the mouthpiece. Do not immerse the metal container in water.
Using a higher dose of Aspulmo than recommended
After taking more inhalations than recommended, you should immediatelyconsult a doctor
or pharmacist for advice.
Stop using Aspulmo if any symptoms of overdose occur
overdose,such as rapid heartbeat, headaches, excessive restlessness (see
section 4: Possible side effects).
It is recommended to administer a medicine that selectively blocks beta-adrenergic receptors in the heart, but these medicines should be used with caution in people with a history of bronchospasm.
Salbutamol overdose may cause a decrease in potassium levels in the blood. The doctor will recommend monitoring potassium levels in the blood serum.
Missing a dose of Aspulmo
Do not take a double dose to make up for a missed dose.
The next dose should be taken according to the recommendations, or earlier, in case of shortness of breath or wheezing.
If you have any further doubts about using this medicine, you should consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Frequent(more than 1 in 100, but less than 1 in 10 patients using the medicine)
side effects related to the use of Aspulmo:
- muscle tremors, headaches,
- rapid heart rate (tachycardia).
Uncommon(more than 1 in 1,000, but less than 1 in 100 patients using the medicine)
side effects related to the use of Aspulmo:
- palpitations,
- irritation of the mucous membrane of the mouth and throat.
Rare(more than 1 in 10,000, but less than 1 in 1,000 patients using the medicine)
side effects related to the use of Aspulmo:
- decrease in potassium levels in the blood,
- peripheral vasodilation,
- muscle cramps.
Very rare(less than 1 in 10,000 patients using the medicine) side effects related to the use of Aspulmo:
- hypersensitivity reactions, including angioedema (limited skin and mucous membrane swelling, including face and throat swelling, which can cause difficulty breathing and swallowing), urticaria, bronchospasm, hypotension, anaphylaxis.
If any of these symptoms occur, you should immediately contact a doctor;
- excessive restlessness,
- heart rhythm disorders (including atrial fibrillation, supraventricular tachycardia, and extrasystoles),
- paradoxical bronchospasm. Like other inhaled medicines, paradoxical bronchospasm may occur, manifested by wheezing immediately after taking the medicine. In such a situation, you should immediately stop taking Aspulmo and consult a doctor. The doctor will assess the patient's condition and, if necessary, prescribe other treatment.
Frequency not known (frequency cannot be estimated from the available data):myocardial ischemia.
If any of the side effects worsen, you should tell your doctor or pharmacist.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Jerozolimskie Avenue 181C, 02-222 Warsaw, phone: +48 22 49 21 301, fax: +48 22 49 21 309,
website: https://smz.ezdrowie.gov.pl.
By reporting side effects, you can help provide more information on the safety of this medicine. Side effects can also be reported to the marketing authorization holder.
5. How to store Aspulmo
Do not store above 30°C.
Store in the original packaging to protect from light.
Pressurized container, do not pierce, do not burn, even if the packaging is empty.
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and container after EXP. The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Aspulmo contains
- The active substance of Aspulmo is salbutamol in the form of salbutamol sulfate. One inhalation dose contains 100 μg (micrograms) of salbutamol (in the form of salbutamol sulfate).
- The other ingredients are: oleic acid, anhydrous ethanol, 1,1,1,2-tetrafluoroethane (HFA 134a).
What Aspulmo looks like and contents of the pack
Aluminum pressurized container closed with a metering valve, equipped with a mouthpiece and protective cap, in a cardboard box. The container contains 200 doses (10 ml suspension).
This medicine contains fluorinated greenhouse gases.
Each inhaler contains 13.05g HFA 134a (1,1,1,2-tetrafluoroethane), which corresponds to 0.0187 tons of CO2 equivalent (GWP = 1430).
Marketing authorization holder and manufacturer
Marketing authorization holder:
Exeltis Poland Sp. z o.o.
Szamocka Street 8
01-748 Warsaw
e-mail: [email protected]
Manufacturer:
Laboratorio Aldo-Unión, S.L.
Baronesa de Maldá, 73
08950 ESPLUGUES DE LL.
Barcelona, Spain
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterLaboratorio Aldo-Union S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AspulmoDosage form: Powder, 100 mcg/doseActive substance: salbutamolManufacturer: Orion CorporationPrescription requiredDosage form: Powder, 200 mcg/doseActive substance: salbutamolManufacturer: Orion CorporationPrescription requiredDosage form: Aerosol, 100 mcg/doseActive substance: salbutamolManufacturer: Aeropharm GmbHPrescription required
Alternatives to Aspulmo in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Aspulmo in Spain
Alternative to Aspulmo in Ukraine
Online doctors for Aspulmo
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Aspulmo – subject to medical assessment and local rules.














