How to use Biodacina ophthalmicum 0,3%
Package Leaflet: Information for the Patient
Biodacyna Ophthalmicum 0.3%, 3 mg/ml, Eye Drops, Solution
Amikacin
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed to you by a doctor. Do not pass it on to others. The medicine may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. See section 4.
Table of Contents of the Leaflet
- 1. What is Biodacyna Ophthalmicum 0.3% and what is it used for
- 2. Important information before using Biodacyna Ophthalmicum 0.3%
- 3. How to use Biodacyna Ophthalmicum 0.3%
- 4. Possible side effects
- 5. How to store Biodacyna Ophthalmicum 0.3%
- 6. Contents of the pack and other information
1. What is Biodacyna Ophthalmicum 0.3% and what is it used for
The medicine is intended for instillation into the eye, as recommended by a doctor.
Biodacyna Ophthalmicum 0.3% eye drops contain the aminoglycoside antibiotic amikacin.
Amikacin is a semi-synthetic antibiotic from the aminoglycoside group with a broad spectrum of activity. It exhibits strong bactericidal activity against most strains resistant to other aminoglycoside antibiotics (gentamicin, tobramycin, and kanamycin). It is active against Gram-negative and some Gram-positive bacteria.
The antibiotic is effective in treating eye infections caused by bacteria: Staphylococcus
aureus, Proteus spp., Escherichia coli, Serratia spp., Enterobacter spp., Klebsiella spp. and
Pseudomonas spp., including Pseudomonas aeruginosa.
Biodacyna Ophthalmicum 0.3% is used to treat bacterial eye infections: conjunctivitis, keratitis, blepharitis, and meibomian gland infection, as well as for the treatment of hordeolum.
The medicine is given prophylactically to patients preparing for eye surgery.
2. Important information before using Biodacyna Ophthalmicum 0.3%
When not to use Biodacyna Ophthalmicum 0.3%:
- if the patient is allergic to amikacin or other aminoglycoside antibiotics, or to any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting treatment with Biodacyna Ophthalmicum 0.3%, consult a doctor or pharmacist.
If the use of the medicine causes disturbing symptoms of eye irritation (itching, burning, or swelling of the eyelids), contact a doctor immediately.
- If the treatment does not improve after several days, consult a doctor.
- Do not use the medicine for longer than recommended by the doctor; prolonged use of the antibiotic may lead to the development of resistant strains and secondary bacterial or fungal infections.
- Do not touch the dropper tip to any surface, as this may cause contamination of the drops. Using contaminated drops can lead to dangerous complications, even vision loss.
- In case of eye infection, wearing contact lenses is not recommended.
- If the patient or their family members have a disease associated with mitochondrial mutation (genetic disease) or hearing loss caused by the use of antibiotic drugs, inform the doctor or pharmacist before taking the aminoglycoside; some mitochondrial mutations may increase the risk of hearing loss after using this medicine. Before administering Biodacyna Ophthalmicum 0.3%, the doctor may recommend genetic testing. Aminoglycoside antibiotics can cause irreversible, partial, or complete hearing loss when administered systemically or when used topically on open wounds or damaged skin. This effect is dose-dependent and increases in case of renal or hepatic insufficiency.
Biodacyna Ophthalmicum 0.3% and other medicines
Tell the doctor or pharmacist about all medicines the patient is currently taking or has recently taken, as well as any medicines the patient plans to take.
In severe infections caused by Pseudomonas aeruginosa, the doctor may recommend amikacin in the form of an injection solution and other eye drops.
When using Biodacyna Ophthalmicum 0.3% eye drops and another eye preparation, wait at least 15 minutes between administrations, so that the drop can spread over the cornea in an undiluted state.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult a doctor or pharmacist before using this medicine.
Driving and using machines
Symptoms of the disease and the occurrence of side effects may make it impossible to drive vehicles and operate machines.
Biodacyna Ophthalmicum 0.3% contains phosphates
The medicine contains 6.34 mg of phosphates in each milliliter of solution. In patients with severe damage to the transparent, anterior part of the eye (cornea), phosphates may, in very rare cases, cause corneal clouding during treatment due to calcium deposition.
Biodacyna Ophthalmicum 0.3% contains benzalkonium chloride
The medicine contains 0.1 mg of benzalkonium chloride in each milliliter of solution. Benzalkonium chloride can be absorbed by soft contact lenses and change their color. Remove contact lenses before instillation and wait at least 15 minutes before reinserting.
In case of eye infection, wearing contact lenses is not recommended.
Benzalkonium chloride can also cause eye irritation, especially in people with dry eye syndrome or corneal disorders (the transparent layer at the front of the eye). If abnormal sensations occur in the eye, stinging, or pain in the eye after using the medicine, consult a doctor.
3. How to use Biodacyna Ophthalmicum 0.3%
Always use this medicine exactly as your doctor or pharmacist has told you. If you are not sure, ask your doctor or pharmacist.
Instill 1-2 drops of the medicine 3 or 4 times a day into the conjunctival sac (by gently pulling down the lower eyelid) usually for 7 to 10 days, as recommended by the doctor.
Method of administration:
- 1. Wash your hands and take a comfortable sitting or standing position.
- 2. Unscrew the cap.
- 3. Do not touch the dropper tip to any surface, as this may cause contamination of the drops. Using contaminated drops can lead to dangerous complications, even vision loss.
- 4. Gently pull down the lower eyelid of the affected eye with your finger.
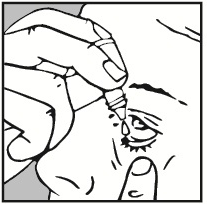
- 5. Hold the upper part of the dropper close to the eye, without touching the eye. Gently squeeze the bottle so that one drop falls into the eye. Make sure not to squeeze the bottle too hard, so that no more than one drop falls into the eye.
- 6. Then release the lower eyelid.
- 7. After using the medicine, close your eyes for as long as possible (3-5 minutes) and press the corner of the affected eye near the nose with your finger. This will prevent the medicine from entering other parts of the body. If the drop did not fall into the eye, instill a second drop.
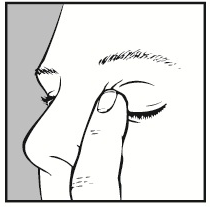
- 8. Close the bottle.
Using more than the recommended dose of Biodacyna Ophthalmicum 0.3%
Overdose may occur if the drops are used more frequently than 4 times a day.
This may cause an increase in side effects.
Stopping the use of Biodacyna Ophthalmicum 0.3%
Do not stop using the medicine without consulting a doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects are very rare (less than 1 in 10,000 people). They usually do not require discontinuation of treatment.
Nonspecific conjunctivitis, burning sensation, itching, irritation, and hypersensitivity reactions with eyelid redness, itching, swelling may occur.
If after using Biodacyna Ophthalmicum 0.3% the patient experiences hypersensitivity reactions, discontinue the use of the medicine and consult a doctor immediately.
In patients with severe damage to the transparent, anterior part of the eye (cornea), phosphates may, in very rare cases, cause corneal clouding during treatment due to calcium deposition.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Pharmacovigilance of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Biodacyna Ophthalmicum 0.3%
Keep the medicine out of the sight and reach of children.
Store in a temperature below 25°C. Protect from light.
The medicine is suitable for use within 28 days of first opening the bottle.
Discard the medicine after completion of treatment.
Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month stated.
The inscription on the packaging after the abbreviation EXP means the expiry date, and after the abbreviation Lot means the batch number.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Biodacyna Ophthalmicum 0.3% contains
- The active substance is amikacin (as sulfate). Each milliliter of solution contains 3 mg of amikacin.
- The excipients are: disodium phosphate dodecahydrate, sodium dihydrogen phosphate dihydrate, sodium chloride, benzalkonium chloride, purified water.
What Biodacyna Ophthalmicum 0.3% looks like and contents of the pack
LDPE bottle with LDPE dropper and HDPE cap in a cardboard box.
The pack contains 5 ml of solution.
Marketing authorization holder
Zakłady Farmaceutyczne POLPHARMA S.A.
ul. Pelplińska 19, 83-200 Starogard Gdański
phone: +48 22 364 61 01
Manufacturer
Jadran-Galenski Laboratorij d.d.
Svilno 20, 51000 Rijeka
Croatia
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterJadran-Galenski laboratorij d.d.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Biodacina ophthalmicum 0,3%Dosage form: Drops, 15 mg/gActive substance: azithromycinManufacturer: Laboratoires Thea Laboratoires UnitherPrescription requiredDosage form: Drops, 15 mg/gActive substance: azithromycinPrescription requiredDosage form: Drops, 15 mg/mlActive substance: azithromycinPrescription required
Alternatives to Biodacina ophthalmicum 0,3% in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Biodacina ophthalmicum 0,3% in Spain
Online doctors for Biodacina ophthalmicum 0,3%
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Biodacina ophthalmicum 0,3% – subject to medical assessment and local rules.