
Baclofen Accord
Ask a doctor about a prescription for Baclofen Accord

How to use Baclofen Accord
Leaflet accompanying the packaging: patient information
Baclofen Accord, 50 micrograms/mL solution for injection
Baclofen Accord, 0.5 mg/mL solution for infusion
Baclofen Accord, 2 mg/mL solution for infusion
Baclofen
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- -Keep this leaflet, so you can read it again if you need to.
- -If you have any doubts, consult your doctor, pharmacist, or nurse.
- -If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor or pharmacist.
Table of contents of the leaflet
- 1. What Baclofen Accord is and what it is used for
- 2. Important information before taking Baclofen Accord
- 3. How to take Baclofen Accord
- 4. Possible side effects
- 5. How to store Baclofen Accord
- 6. Package contents and other information
1. What Baclofen Accord is and what it is used for
Baclofen Accord is administered by injection or continuous infusion into the spinal canal, directly into the cerebrospinal fluid (intrathecal injection), and relieves severe muscle stiffness (spasticity). The first injection is in the form of a bolus, and then the medicine is administered by infusion. Baclofen Accord is used to treat severe, long-term muscle tension (spasticity) that occurs in various diseases, such as:
- -brain or spinal cord injuries or diseases,
- -multiple sclerosis, which is a progressive disease of the brain and spinal cord, with physical and mental symptoms.
Baclofen Accord is used in adults and children over 4 years of age. It is used when other oral medications, including baclofen, have been ineffective or have caused unacceptable side effects.
2. Important information before taking Baclofen Accord
When not to take Baclofen Accord
- -If the patient is allergic to baclofen or any of the other ingredients of Baclofen Accord (listed in section 6).
- -If the patient has epilepsy that is resistant to treatment.
- -In case of administration by a route other than into the spinal canal.
Warnings and precautions
Before starting treatment with Baclofen Accord, the patient should discuss the following with their doctor, pharmacist, or nurse:
- -if the patient has any infection,
- -if the patient has had a head injury. In patients with spasticity caused by head injury, it is recommended not to start treatment with Baclofen Accord until the symptoms of spasticity have stabilized and can be reliably assessed.
- -if the patient has had autonomic dysreflexia, i.e., a nervous system reaction to excessive stimulation, causing a sudden, very high increase in blood pressure,
- -if the patient has decreased circulation of cerebrospinal fluid due to impaired flow, for example, due to inflammation or injury,
- -if the patient has curable epilepsy,
- -if the patient has had stomach or intestinal ulcers;
- -if the patient has excessive bladder sphincter activity,
- -if the patient has acute or chronic confusional states,
- -if the patient has psychotic or schizophrenic disorders (mental illness),
- -if the patient has Parkinson's disease,
- -if the patient has impaired kidney or liver function,
- -if the patient has insufficient blood flow to the brain (cerebrovascular insufficiency),
- -if the patient has heart or breathing difficulties, monitoring of heart and respiratory function is necessary in the initial phase of the study, especially if the patient has heart or breathing difficulties.
The patient should immediately contact their doctor if they experience any of the following symptoms during treatment with Baclofen Accord:
- -If the patient experiences back, neck, and buttock pain (this may be a symptom of a type of spinal deformity called scoliosis).
If the patient thinks that Baclofen Accord is not working as well as usual, they should immediately contact their doctor. It is essential to ensure that there are no problems with the pump. The patient will be closely monitored in a fully equipped environment with personnel during the screening phase and dose adjustment immediately after pump implantation. The patient will be regularly evaluated for dosage requirements, possible side effects, or signs of infection. The functioning of the delivery system will also be checked. The patient should not miss hospital visits related to pump refilling.
Children and adolescents
Baclofen Accord should not be used in children under 4 years of age. Older children must have sufficient body weight to accommodate the implanted pump. Clinical data on the use of the medicine in children under four years of age are limited.
Elderly
Some patients over 65 years of age have been treated with intrathecal baclofen without particular problems during clinical trials. However, experience with oral baclofen tablets shows that this group of patients may be more susceptible to side effects. Therefore, elderly patients should be closely monitored for side effects.
Baclofen Accord and other medicines
The patient should tell their doctor or nurse about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. If the patient is taking any of the following medicines, they should discuss this with their doctor, as they may affect Baclofen Accord or Baclofen Accord may interact with these medicines:
- -Other medicines used to treat muscle spasms. If possible, the doctor may slowly stop other medicines used to treat muscle spasms.
- -Medicines used to treat depression.
- -Medicines used to treat high blood pressure.
- -Levodopa, carbidopa: medicines used to treat Parkinson's disease.
- -Strong painkillers, such as morphine.
- -Medicines that slow down the central nervous system, such as sleeping pills.
- -Other medicines administered into the spinal canal.
- -It is not recommended to administer other medicines into the spinal canal during treatment with Baclofen Accord.
Concomitant use of general anesthetics may increase the risk of cardiac arrhythmias and seizures.
Baclofen Accord and alcohol
The patient should avoid consuming alcohol during treatment with Baclofen Accord, as this may lead to unwanted intensification or unpredictable change in the effect of the medicine.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before taking Baclofen Accord. PregnancyExperience with intrathecal baclofen during pregnancy is limited. Baclofen Accord should not be used during pregnancy unless the expected benefits to the mother outweigh the potential risk to the fetus. BreastfeedingBaclofen Accord passes into breast milk, but after intrathecal administration, a low concentration is expected. Therefore, Baclofen Accord can be used during breastfeeding.
Driving and using machines
During treatment with Baclofen Accord, the ability to drive and use machines may be significantly impaired. In some people, Baclofen Accord may cause drowsiness, dizziness, double vision, difficulty controlling movements, or hallucinations. The patient should not drive or operate machinery until these symptoms have resolved, if they occur. Before driving or operating machinery, the patient should consult their doctor.
Baclofen Accord contains sodium chloride:
Baclofen Accord, 50 micrograms/mL solution for injection, contains less than 1 mmol of sodium (23 mg) per 1 mL, which means that the medicine is essentially "sodium-free". Baclofen Accord, 0.5 mg/mL solution for infusion, contains 70.78 mg of sodium in 20 mL, which corresponds to 3.5% of the WHO-recommended maximum daily intake of sodium, which is 2 g for an adult. Baclofen Accord, 2 mg/mL solution for infusion, contains less than 1 mmol of sodium (23 mg) per 5 mL, which means that the medicine is essentially sodium-free. Baclofen Accord, 2 mg/mL solution for infusion, contains 70.78 mg of sodium in 20 mL, which corresponds to 3.5% of the WHO-recommended maximum daily intake of sodium, which is 2 g for an adult.
3. How to take Baclofen Accord
This medicine should always be taken exactly as prescribed by the doctor or pharmacist. If the patient has any doubts, they should consult their doctor or pharmacist. Only a doctor can change the dose of the medicine. Baclofen Accord can only be administered by a specially qualified doctor.
Usual dose
The dose depends on the patient's condition. The doctor will determine the dose after examining the patient's reaction to the medicine. First, the doctor will give the patient a single dose of Baclofen Accord to check if it is suitable for them. During this period, heart and lung function will be closely monitored. If the symptoms improve, a special pump will be implanted in the chest or abdominal wall, which will continuously administer Baclofen Accord. The doctor will provide the patient with all necessary instructions for using the pump and information about dosing. The patient should make sure they understand everything. The dose depends on the patient's reaction to the medicine. Treatment starts with a small dose, which is gradually increased over several days under the doctor's supervision until the target dose is reached. Side effects are more likely if the initial dose is too high or the dose is increased too quickly. To avoid these reactions, which can be serious, it is essential to ensure that the pump does not run out. The patient should not miss hospital visits.
It is extremely important to have regular check-ups with the doctor to refill the pump.
Otherwise, muscle spasms may return due to insufficient doses of Baclofen Accord.
This can lead to increased muscle spasms.
If muscle spasticity does not improve or if spasms start to recur, gradually or suddenly, the patient should immediately contact their doctor.
Discontinuation of Baclofen Accord,
It is crucial that the patient and their caregivers can recognize the symptoms of Baclofen Accord withdrawal. These may occur suddenly or gradually, for example, due to malfunction of the pump due to battery problems, catheter problems, or alarm malfunction. Withdrawal symptoms include:
- -Increased spasticity, excessive muscle tension
- -Difficulty moving muscles.
- -Increased heart rate or pulse.
- -Itching, tingling, burning, or numbness (paresthesia) of the hands or feet
- -Palpitations
- -Anxiety
- -High body temperature
- -Low blood pressure
- -Mental disorders, such as agitation, confusion, hallucinations, thought and behavioral disorders, seizures
- -Prolonged painful erection (priapism)
If the patient experiences any of the above symptoms, they should immediately inform their doctor.
If the patient does not receive immediate treatment, more severe side effects may occur after these symptoms.
Method of administration
Baclofen Accord can only be administered into the spinal canal (intrathecal administration).
Duration of treatment
To be determined by the doctor. In some patients, during long-term treatment with Baclofen Accord, the medicine may become less effective. To prevent this, the doctor may recommend occasional breaks in treatment.
Overdose of Baclofen Accord
It is essential that the patient and their caregivers can recognize the symptoms of Baclofen Accord overdose. This may occur if the pump does not work correctly. The patient should immediately inform their doctorif they experience any of the following symptoms:
- -Unusual muscle weakness
- -Drowsiness, confusion, or loss of consciousness
- -Dizziness, fainting
- -Excessive salivation, abnormally low body temperature
- -Nausea or vomiting
- -Breathing difficulties, respiratory arrest
- -Seizures
Discontinuation of Baclofen Accord
If it is necessary to discontinue treatment, only a doctor can do this by gradually reducing the dose to avoid side effects. Abrupt discontinuation of intrathecal Baclofen Accord may cause withdrawal symptoms, which in some cases have been fatal. If the patient has any further doubts about the use of this medicine, they should consult their doctor or nurse.
4. Possible side effects
Like all medicines, Baclofen Accord can cause side effects, although not everybody gets them. It is also known that many of these side effects are related to the underlying disease for which the patient is being treated. Side effects may occur with the following frequency: Very common(may affect more than 1 in 10 people)
- -drowsiness
- -decreased muscle tone
Common(may affect up to 1 in 10 people)
- -seizures
- -sedation, dizziness, feeling of emptiness in the head
- -pain, fever, chills
- -abnormal skin sensations, such as tingling or numbness (paresthesia)
- -vision problems, such as blurred or double vision
- -slurred speech
- -lethargy (feeling of extreme fatigue and lack of energy), weakness
- -breathing difficulties (respiratory depression, dyspnea, slow breathing), pneumonia (aspiration pneumonia)
- -insomnia
- -confusion, disorientation, anxiety, restlessness, depression
- -sudden drop in blood pressure when changing position from lying down to sitting or from sitting to standing (orthostatic hypotension)
- -constipation, diarrhea
- -dry mouth, decreased appetite, excessive salivation
- -rash, itching
- -swelling of facial, hand, or foot tissues
- -urinary incontinence
- -increased muscle tone, muscle weakness
- -sexual disorders, such as impotence
- -seizures
- -headache
- -nausea
- -vomiting
- -difficulty urinating
The following side effects occur more frequently in patients with spasticity of cerebral origin: seizures, headaches, nausea, vomiting, and difficulty urinating Uncommon(may affect up to 1 in 100 people)excessive feeling of coldness involuntary eye movements (nystagmus) difficulty controlling movements (ataxia) memory impairment mood disorders, euphoria, paranoia, hallucinations, suicidal thoughts and attempts intestinal obstruction, difficulty swallowing, loss of taste, dehydration high blood pressure, slow heart rate deep vein thrombosis red or pale skin, excessive sweating hair loss Rare(may affect up to 1 in 1,000 people)life-threatening withdrawal symptoms due to problems with drug administration Frequency not known(frequency cannot be estimated from the available data)worsening of spinal curvature (scoliosis) inability to achieve or maintain an erection (erectile dysfunction) Description of withdrawal symptoms, see "Discontinuation of Baclofen Accord". Description of overdose symptoms, see "Overdose of Baclofen Accord". There have been reports of problems with the pump and delivery system, such as infections, meningitis, or inflammation at the tip of the catheter.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor, pharmacist, or nurse. Side effects can also be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocides of the Office for Registration of Medicinal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: 22 49-21-301, fax: 22 49-21-309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Baclofen Accord
The medicine should be stored out of sight and reach of children. There are no special precautions for storing the medicine. Do not use this medicine after the expiry date stated on the carton and ampoule/vial after EXP. The expiry date refers to the last day of the month stated. After opening/dilution: use immediately. Since the use of the medicine is limited to hospital use, the disposal of unused medicine is the responsibility of the hospital.
6. Package contents and other information
What Baclofen Accord contains
- The active substance of the medicine is baclofen.
Baclofen Accord, 50 micrograms/mL solution for injection: 1 ampoule with 1 mL of solution for injection contains 0.05 mg of baclofen. Baclofen Accord, 0.5 mg/mL solution for infusion: 1 mL of solution for infusion contains 0.5 mg of baclofen. 1 vial with 20 mL of solution for infusion contains 10 mg of baclofen. Baclofen Accord, 2 mg/mL solution for infusion: 1 mL of solution for infusion contains 2.0 mg of baclofen. 1 ampoule with 5 mL of solution for infusion contains 10 mg of baclofen. 1 vial with 20 mL of solution for infusion contains 40 mg of baclofen. The other ingredients are sodium chloride, water for injections.
What Baclofen Accord looks like and contents of the pack
Baclofen Accord is available in four different sizes containing baclofen in amounts of 50 micrograms in 1 mL, 10 mg in 20 mL, 10 mg in 5 mL, and 40 mg in 20 mL. Baclofen Accord, 50 micrograms/mL solution for injection: Baclofen Accord is a clear, colorless solution in a 1 mL ampoule made of transparent type I glass. Each pack contains 1 or 5 ampoules. Baclofen Accord, 0.5 mg/mL solution for infusion: Baclofen Accord is a clear, colorless solution in a 20 mL vial made of clear type I glass with a gray aluminum flip-off seal. Each pack contains 1 vial. Baclofen Accord, 2 mg/mL solution for infusion: Baclofen Accord is a clear, colorless solution in a 5 mL ampoule made of transparent type I glass. Each pack contains 1, 5, or 10 ampoules. Baclofen Accord is a clear, colorless solution in a 20 mL vial made of clear type I glass with a green aluminum flip-off cap. Each pack contains 1 vial. Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
Accord Healthcare Polska Sp.z o.o., ul. Taśmowa 7, 02-677 Warsaw, Tel: +48 22 577 28 00
Manufacturer/Importer
Accord Healthcare Polska Sp.z o.o., ul. Lutomierska 50, 95-200 Pabianice
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
| Member State | Medicinal product name |
| Denmark | Baclofen Accord |
| Norway | Baclofen Accord |
| Poland | Baclofen Accord |
| Sweden | Baclofen Accord |
Date of last revision of the leaflet: -------------------------------------------------------------------------------------------------------------------------------------
The following information is intended for healthcare professionals only.
7. INFORMATION FOR HEALTHCARE PROFESSIONALS ABOUT
DILUTION OF BACLOFEN ACCORD FOR INJECTION AND INFUSION
Baclofen Accord is intended for intrathecal administration or continuous infusion. The medicine should not be mixed with other infusion or injection solutions. Ampoules are for single use only. Unused solution should be discarded. After opening and/or dilution: use immediately. The solution should only be used if it is clear and colorless and free of particles. Information on dosing, administration, and other information can be found in the Summary of Product Characteristics and the patient information leaflet. The medicine should not be administered by any route other than intrathecally. Baclofen Accord should not be administered intravenously, intramuscularly, subcutaneously, or epidurally. Only pumps made of material compatible with the product and equipped with a built-in antibacterial filter should be used. Stages of testing, implantation, and dose adjustment during intrathecal administrationshould be performed in a hospital setting, in centers with special experience, under close medical supervision by qualified doctors.Due to the possibility of life-threatening or serious side effects, immediate intensive medical care should be ensured.
Instructions for dilution
In the case of patients requiring concentrations other than 50 micrograms/mL, 0.5 mg/mL, and 2 mg/mL, Baclofen Accord should be diluted with sterile isotonic sodium chloride solution for injection without preservatives. Dilution should be performed under aseptic conditions.
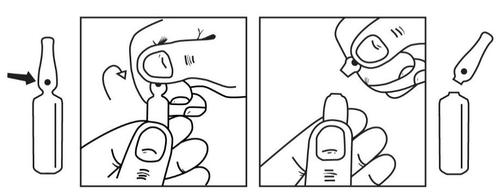
Method of opening ampoules
Place your thumb on the blue dot and break (the break point is located below the blue dot)
- Country of registration
- Active substance
- Prescription requiredNo
- ImporterAccord Healthcare Polska Sp. z o.o.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Baclofen AccordDosage form: Solution, 50 mcg/mlActive substance: baclofenPrescription not requiredDosage form: Solution, 2 mg/mlActive substance: baclofenPrescription not requiredDosage form: Tablets, 25 mgActive substance: baclofenManufacturer: Zakłady Farmaceutyczne POLPHARMA S.A. Zakłady Farmaceutyczne POLPHARMA S.A. Oddział Medana w SieradzuPrescription required
Alternatives to Baclofen Accord in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Baclofen Accord in Ukraine
Alternative to Baclofen Accord in Spain
Online doctors for Baclofen Accord
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Baclofen Accord – subject to medical assessment and local rules.














