
LOCETAR 50 MG/ML MEDICINAL NAIL POLISH

How to use LOCETAR 50 MG/ML MEDICINAL NAIL POLISH
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Locetar 50 mg/ml Medicinal Nail Varnish
Amorolfine
Read the entire package leaflet carefully before starting to use this medicine, as it contains important information for you.
Follow the instructions for administration of the medicine contained in this package leaflet or as indicated by your pharmacist.
- Keep this package leaflet, as you may need to read it again.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this package leaflet. See section 4.
- You should consult a doctor if your symptoms worsen or do not improve after 3 months.
Contents of the Package Leaflet
- What Locetar Medicinal Nail Varnish is and what it is used for.
- What you need to know before starting to use Locetar Medicinal Nail Varnish.
- How to use Locetar Medicinal Nail Varnish.
- Possible side effects.
- Storage of Locetar Medicinal Nail Varnish.
- Contents of the pack and further information.
1. What Locetar Medicinal Nail Varnish is and what it is used for
Locetar contains amorolfine as the active ingredient, which belongs to a group of medicines known as antifungals (used to treat infections caused by fungi and yeasts).
This medicine is indicated for the treatment of fungal infections of the nails, of mild to moderate severity, in adults.
You should consult a doctor if your symptoms worsen or do not improve after 3 months.
2. What you need to know before using Locetar Medicinal Nail Varnish
A fungal infection is considered mild to moderate if it affects the nail plate (without involving the nail matrix) and is characterized by discoloration of the nail (white, yellow, or brown) and thickening, although the appearance may vary considerably.
If the infection is limited to the upper part of the nail, as shown in image 1 or 2, where there is no involvement of the nail matrix, only discoloration (white, yellow, or brown), consult your pharmacist.
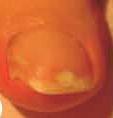
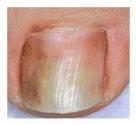


If the infection appears as in photo 3 or 4, where involvement of the nail matrix and nail breakage can be seen, consult your doctor.

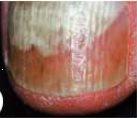


Do not use Locetar Medicinal Nail Varnish
- if you are allergic to amorolfine or any of the other components of this medicine (listed in section 6).
Children and Adolescents
This medicine is not recommended for use in children and adolescents (from 12 to 18 years) due to insufficient clinical experience in this age group.
Warnings and Precautions
Consult your doctor or pharmacist before starting to use this medicine.
- If you have a history of diabetes, immunological disorders (diseases that reduce the body's defenses), peripheral vascular disorders, wounds, pain in the nails, or severely damaged nails, skin conditions such as psoriasis, or any chronic skin condition, edema, or respiratory disorders (yellow nail syndrome).
- If you are diabetic, be careful when cutting your nails.
- If you develop sensitivity to the product, stop treatment and consult your doctor.
- During treatment, do not use artificial nails.
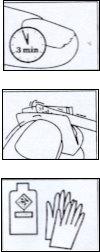
- After applying Locetar, wait at least 10 minutes before applying any cosmetic nail polish.
- Before reapplying Locetar, remove any cosmetic nail polish carefully.
- In patients undergoing treatment who regularly work with organic solvents, it is recommended to wear impermeable gloves to protect the hands.
- Avoid contact with eyes and mucous membranes. If accidental contact with the eyes occurs, rinse with plenty of water and consult an ophthalmologist if necessary.
This medicine may cause allergic reactions, some of which can be serious. If this occurs, discontinue treatment, remove the medicine immediately with a nail polish remover, and seek medical advice. The medicine should not be reapplied.
You should seek urgent medical attention if you experience any of the following symptoms:
- Difficulty breathing.
- Your face, lips, tongue, or throat are swollen.
- Your skin has developed a severe rash.
Using Locetar Medicinal Nail Varnish with other medicines
Inform your doctor or pharmacist if you are using, have recently used, or may need to use any other medicine.
Pregnancy and Breastfeeding
Pregnancy
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine. Treatment with Locetar will only be carried out if necessary, after the responsible doctor has carefully evaluated the benefits versus the possible risks.
Breastfeeding
It is not known whether it is safe to apply this medicine to breastfeeding women. Treatment with Locetar will only be carried out if necessary, after the responsible doctor has carefully evaluated the benefits versus the possible risks.
Driving and Using Machines
The influence of this medicine on the ability to drive or use machines is negligible or nonexistent.
Locetar contains alcohol (ethanol)
This medicine contains 481.3 mg of alcohol (ethanol) per milliliter of solution. It may cause a burning sensation on damaged skin.
Locetar contains ethanol, which is flammable and should not be used near an open flame, lit cigarettes, or certain devices (e.g., hair dryers).
3. How to use Locetar Medicinal Nail Varnish
Follow the instructions for administration of this medicine contained in this package leaflet or as indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
Method of administration: cutaneous use (only for use on the nails, not to be applied to the surrounding skin).
Locetar should only be used by adults.
Apply the solution 1 or 2 times a week, only to the affected nails of the hands or feet. Try to associate the use of the medicinal nail varnish with a hygiene habit, on 1 day of the week that you choose, and maintain this routine while the treatment lasts.
The required duration of treatment will depend mainly on the severity and location of the infection. It is usually 6 months for fingernails and 9 to 12 months for toenails.
If symptoms worsen or new symptoms appear, the clinical situation should be reevaluated. If no improvement is observed after 3 months of treatment, consult a doctor.
 Method of application:
Method of application:

Attention:
Healthy nails should never be filed with the same file used for infected nails. Fungal infections are contagious. To prevent infection, avoid letting another person use the same file.

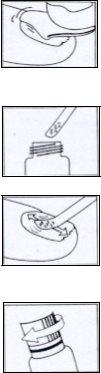

General hygiene measures should be applied to avoid the appearance of other infections or relapses.
Regular trimming of the nails is recommended to eliminate the infected parts of the nail.
In the case of coexistence of tinea pedis, it should be treated with an appropriate antifungal cream.
In case of doubt, consult a doctor or pharmacist.
If you use more Locetar Medicinal Nail Varnish than you should
This medicine is not to be ingested. IT IS FOR EXTERNAL USE ONLY.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately, or go to a medical center, or call the Toxicology Information Service, phone: 91 5620420, indicating the medicine and the amount ingested.
If you forget to use Locetar Medicinal Nail Varnish
Do not apply a double dose to make up for forgotten doses.
If you interrupt treatment with Locetar Medicinal Nail Varnish
If you interrupt treatment with Locetar before your nail(s) are clean or almost clean, the fungi may not have disappeared. In this case, the condition of your nails may worsen again.
If you have any other doubts about the use of this medicine, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Adverse reactions are rare. They may include nail changes (e.g., nail discoloration, nail breakage, and brittle nails). These reactions may also be related to the onychomycosis itself.
Rare (may affect up to 1 in 1,000 people)
- Nail change,
- Nail discoloration,
- Onychoclasis (nail breakage),
- Onychorexis (brittle nails).
Very rare (may affect up to 1 in 10,000 people)
- Burning sensation on the skin.
Frequency not known (cannot be estimated from the available data):
- Systemic allergic reaction (a serious allergic reaction that may be associated with swelling of the face, lips, tongue, or throat, difficulty breathing, and/or severe skin rash).
- Erythema, itching, contact dermatitis, urticaria, and blisters.
Reporting of Side Effects
If you experience any side effects, consult your doctor or pharmacist, even if they are side effects not listed in this package leaflet. You can also report them directly through the Spanish Medicines and Healthcare Products Agency's website: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Locetar Medicinal Nail Varnish
Keep this medicine out of the sight and reach of children.
No special storage conditions are required.
Store in the original packaging.
Do not use this medicine after the expiry date stated on the packaging after EXP. The expiry date is the last day of the month indicated.
No special storage conditions are required.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in the pharmacy's SIGRE collection point. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the Pack and Further Information
Locetar Medicinal Nail Varnish Composition
- The active ingredient is amorolfine. Each milliliter of solution contains 50 milligrams of amorolfine.
- The other excipients are methacrylic acid copolymer, triacetin, butyl acetate, ethyl acetate, and 96% ethanol.
Appearance of the Product and Contents of the Pack
Ambber glass bottle with a polypropylene (HDPE) cap, containing 5 ml of solution.
The packaging includes: 30 cleaning wipes, in individual sachets, as well as 10 disposable spatulas and 30 nail files.
Ambber glass bottle with a polypropylene (HDPE) cap, containing 5 ml of solution, and a spatula integrated into the cap (LDPE).
The packaging includes 30 cleaning wipes, in individual sachets, and 30 nail files.
Only one of the packaging options may be marketed.
Marketing Authorization Holder
Laboratorios Galderma, S.A.
Serrano Galvache, 56
28033 Madrid
Spain
Manufacturer
Laboratoires Galderma
Alby sur Chéran (France)
Date of Last Revision of this Package Leaflet:October 2017
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.es/
.
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LOCETAR 50 MG/ML MEDICINAL NAIL POLISHDosage form: NAIL POLISH, 50 mg/mlActive substance: amorolfineManufacturer: Bluefish Pharmaceuticals Ab (Publ)Prescription requiredDosage form: NAIL POLISH, 5% w/w Amorolfine (as hydrochloride)Active substance: amorolfineManufacturer: Isdin S.A.Prescription requiredDosage form: NAIL POLISH, 5% w/w Amorolfine (as hydrochloride)Active substance: amorolfineManufacturer: Laboratorio Stada S.L.Prescription required
Online doctors for LOCETAR 50 MG/ML MEDICINAL NAIL POLISH
Discuss questions about LOCETAR 50 MG/ML MEDICINAL NAIL POLISH, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions








