
NUWIQ 1500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use NUWIQ 1500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
- PACKAGE LEAFLET
Package Leaflet: Information for the User
Nuwiq 250 IU powder and solvent for solution for injection
Nuwiq 500 IU powder and solvent for solution for injection
Nuwiq 1000 IU powder and solvent for solution for injection
Nuwiq 1500IU powder and solvent for solution for injection
Nuwiq 2000 IU powder and solvent for solution for injection
Nuwiq 2500 IU powder and solvent for solution for injection
Nuwiq 3000 IU powder and solvent for solution for injection
Nuwiq 4000 IU powder and solvent for solution for injection
turoctocog alfa (recombinant human coagulation factor VIII)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor.
- This medicine has been prescribed for you only, and you should not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Nuwiq and what is it used for
- What you need to know before you use Nuwiq
- How to use Nuwiq
- Possible side effects
- Storage of Nuwiq
- Contents of the pack and other information
1. What is Nuwiq and what is it used for
Nuwiq contains the active substance recombinant human coagulation factor VIII (turoctocog alfa). Factor VIII is necessary for blood to clot and stop bleeding. In patients with haemophilia A (congenital factor VIII deficiency), factor VIII is missing or does not work properly.
Nuwiq replaces the missing factor VIII and is used to treat and prevent bleeding in patients with haemophilia A and can be used in all age groups.
2. What you need to know before you use Nuwiq
Do not use Nuwiq:
- if you are allergic to the active substance turoctocog alfa or any of the other ingredients of this medicine (listed in section 6).
If you are not sure, ask your doctor.
Warnings and precautions
Talk to your doctor before you start using Nuwiq.
There is a rare possibility that you may experience an allergic reaction (a sudden severe allergic reaction) to Nuwiq. You should be able to recognise the early signs of allergic reactions, which are listed in section 4 "Allergic reactions".
If any of these symptoms occur, stop the injection immediately and contact your doctor.
The formation of inhibitors (antibodies) is a known complication that can occur during treatment with all factor VIII medicines. These inhibitors, especially in large quantities, can prevent the treatment from working properly, so you and your child will be carefully monitored for the development of such inhibitors. If your bleeding or your child's bleeding is not controlled with Nuwiq, contact your doctor immediately.
Cardiovascular events
In patients with cardiovascular risk factors, replacement therapy with FVIII may increase the risk of cardiovascular events.
Complications associated with catheters
If you require a central venous access device (CVAD), the risk of CVAD-associated complications, including localised infections, bacteria in the blood, and thrombosis at the catheter implantation site, should be considered.
It is important to keep a record of the batch number of Nuwiq. Therefore, each time you receive a new pack of Nuwiq, note the date and batch number (indicated on the pack after Batch) and keep this information in a safe place.
Other medicines and Nuwiq
Tell your doctor if you are using, have recently used or might use any other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before using this medicine.
Driving and using machines
Nuwiq does not affect the ability to drive and use machines.
Nuwiq contains sodium
This medicine contains 18.4 mg of sodium (a major component of cooking/table salt) in each vial. This is equivalent to 0.92% of the maximum recommended daily intake of sodium for an adult.
3. How to use Nuwiq
Treatment with Nuwiq will be started by a doctor who has experience in the care of patients with haemophilia A. Follow exactly the instructions for administration of this medicine given by your doctor or nurse. If you are unsure, ask your doctor or nurse again.
Nuwiq is usually injected into a vein (intravenously) by your doctor or a nurse with experience in the care of patients with haemophilia A. You or another person may also inject Nuwiq, but only after you have received appropriate training.
Your doctor will calculate your dose of Nuwiq (in International Units = IU) based on your condition and body weight and whether it is being used for prevention or treatment of bleeding. How often you need an injection will depend on how well Nuwiq works for you. Normally, treatment of haemophilia A is lifelong.
Prevention of bleeding
The usual dose of Nuwiq is 20 to 40 IU per kg body weight, administered every 2 to 3 days. However, in some cases, especially in younger patients, more frequent injections or higher doses may be needed.
Treatment of bleeding
The dose of Nuwiq is calculated based on your body weight and the factor VIII levels that need to be achieved. The target factor VIII levels will depend on the severity and location of the bleeding.
If you think that the effect of Nuwiq is too weak, talk to your doctor. Your doctor will do the relevant laboratory tests to make sure you have adequate factor VIII levels. This is especially important if you are going to have major surgery.
Patients who develop factor VIII inhibitors
If your plasma factor VIII level does not reach the expected level with Nuwiq, or if bleeding is not adequately controlled, it may be due to the development of factor VIII inhibitors. Your doctor will check this. You may need a higher dose of Nuwiq or a different product to control bleeding. Do not increase the total dose of Nuwiq to control your bleeding without talking to your doctor.
Use in children and adolescents
The way Nuwiq is used in children and adolescents does not differ from the way it is used in adults. Since it may be necessary to administer factor VIII medicines more frequently in children and adolescents, a central venous access device (CVAD) may be needed. A CVAD is an external connector that allows access to the bloodstream through a catheter without injection through the skin.
If you use more Nuwiq than you should
No symptoms of overdose have been reported. If you have injected more Nuwiq than you should, talk to your doctor.
If you forget to use Nuwiq
Do not take a double dose to make up for forgotten doses. Proceed with the next dose as planned and follow your doctor's recommendations.
If you stop using Nuwiq
Do not stop using Nuwiq without talking to your doctor.
If you have any further questions on the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop using this medicine and seek urgent medical attention if:
- you notice symptoms of allergic reactions
Allergic reactions can include skin rash, hives, itching (urticaria), including generalised urticaria, swelling of the lips and tongue, difficulty breathing, wheezing, chest tightness, vomiting, agitation, low blood pressure, and dizziness. These symptoms can be early signs of anaphylactic shock. If severe allergic reactions (anaphylactic reactions) (very rare: may affect up to 1 in 10,000 people) occur, stop the injection immediately and contact your doctor. Severe symptoms require urgent emergency treatment.
- you notice that the medicine is not working properly (bleeding does not stop or becomes frequent)
In children and adolescents who have not been treated previously with factor VIII medicines, inhibitory antibodies (see section 2) may occur very frequently (more than 1 in 10 patients).
However, in patients who have been treated previously with factor VIII (more than 150 days of treatment), the risk is uncommon (less than 1 in 100 patients). If this happens, the medicines you or your child are taking may stop working properly, and you or your child may experience persistent bleeding. In this case, contact your doctor immediately.
Common side effects that may affect up to 1 in 10 people
Hypersensitivity, fever.
Uncommon side effects that may affect up to 1 in 100 people
Tingling or numbness (paraesthesia), headache, dizziness, vertigo, shortness of breath, dry mouth, back pain, swelling and/or pain at the injection site, a vague feeling of being unwell (malaise), haemorrhagic anaemia, positive test results for non-neutralising antibody formation (in previously treated patients).
Reporting of side effects
If you experience any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Nuwiq
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the label of the vial after EXP. The expiry date is the last day of the month stated.
Store in a refrigerator (2°C to 8°C). Do not freeze. Keep the vial in the outer carton in order to protect from light.
Before Nuwiq powder is reconstituted, it can be stored at room temperature (up to 25°C) for a single period not exceeding 1 month. Note the date when you start storing Nuwiq at room temperature on the carton. Do not store Nuwiq in the refrigerator again after it has been stored at room temperature.
Use the reconstituted solution immediately after reconstitution.
Warnings regarding visible signs of deterioration
Do not use this medicine if you notice visible signs of deterioration of the pack, especially the syringe and/or the vial.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Nuwiq Composition
Powder:
- The active ingredient is human recombinant coagulation factor VIII (simoctocog alfa).
Each powder vial contains 250, 500, 1000, 1500, 2000, 2500, 3000, or 4000 IU of simoctocog alfa. Each reconstituted solution contains approximately 100, 200, 400, 600, 800, 1000, 1200, or 1600 IU/mL of simoctocog alfa.
- The other components are sucrose, sodium chloride, calcium chloride dihydrate, arginine hydrochloride, sodium citrate dihydrate, and poloxamer 188. See section 2, "Nuwiq contains sodium".
Solvent:
Water for injections
Product Appearance and Container Contents
Nuwiq is supplied as a powder and solvent for solution for injection. The powder is white or almost white in a glass vial. The solvent is water for injections in a pre-filled glass syringe.
After reconstitution, the solution is clear, colorless, and free from foreign particles.
Each Nuwiq container contains:
- 1 vial of powder with 250, 500, 1000, 1500, 2000, 2500, 3000, or 4000 IU of simoctocog alfa
- 1 pre-filled syringe with 2.5 mL of water for injections
- 1 vial adapter
- 1 butterfly needle
- 2 alcohol swabs
Marketing Authorization Holder and Manufacturer
Octapharma AB, Lars Forssells gata 23, 112 75 Stockholm, Sweden
For further information about this medicinal product, please contact the local representative of the marketing authorization holder:
Belgium Octapharma Benelux (Belgium) Tel: +32 2 3730890 | Lithuania Octapharma Nordic AB (Sweden) Tel: +46 8 56643000 |
Bulgaria Octapharma Nordic AB (Sweden) Tel: +46 8 56643000 | Luxembourg Octapharma Benelux (Belgium) Tel: +32 2 3730890 |
Czech Republic Octapharma CZ s.r.o. Tel: +420 266 793 510 | Hungary Octapharma Nordic AB (Sweden) Tel: +46 8 56643000 |
Denmark Octapharma Nordic AB (Sweden) Tel: +46 8 56643000 | Malta Octapharma Nordic AB (Sweden) Tel: +46 8 56643000 |
Germany Octapharma GmbH Tel: +49 2173 9170 | Netherlands Octapharma Benelux (Belgium) Tel: +32 2 3730890 |
Estonia Octapharma Nordic AB (Sweden) Tel: +46 8 56643000 | Norway Octapharma AS Tel: +47 63988860 |
Greece Octapharma Hellas SA Tel: +30 210 8986500 | Austria Octapharma Handelsgesellschaft m.b.H. Tel: +43 1 610321222 |
Spain Octapharma S.A. Tel: +34 91 6487298 | Poland Octapharma Poland Sp. z o.o. Tel: +48 22 2082734 |
France Octapharma France Tel: +33 1 41318000 | Portugal Octapharma Produtos Farmacêuticos Lda. Tel: +351 21 8160820 |
Croatia Octapharma Nordic AB (Sweden) Tel: +46 8 56643000 | Romania Octapharma Nordic AB (Sweden) Tel: +46 8 56643000 |
Ireland Octapharma AB (Sweden) Tel: +46 8 56643000 | Slovenia Octapharma Nordic AB (Sweden) Tel: +46 8 56643000 |
Iceland Octapharma AS (Norway) Tel: +47 63988860 | Slovakia Octapharma AG, o.z.z.o. Tel: +421 2 54646701 |
Italy Kedrion S.p.A. Tel: +39 0583 767507 | Finland Octapharma Nordic AB Tel: +358 9 85202710 |
Cyprus Octapharma Nordic AB (Sweden) Tel: +46 8 56643000 | Sweden Octapharma Nordic AB Tel: +46 8 56643000 |
Latvia Octapharma Nordic AB (Sweden) Tel: +46 8 56643000 | United Kingdom (Northern Ireland) Octapharma Limited Tel: +44 161 8373770 |
Date of Last Revision of this Leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
---------------------------------------------------------------------------------------------------------------------------
This information is intended for healthcare professionals only:
On-demand treatment
The dose to be administered and the frequency of administration should always be oriented towards clinical efficacy in each individual case.
In the case of the following hemorrhagic episodes, factor VIII activity should not be below the given plasma activity level (as a percentage of normal or IU/dL) during the corresponding period. The following table can be used as a dosing guide in surgery and hemorrhagic episodes.
Grade of Hemorrhage / Type of Surgical Procedure | Required Factor VIII Level (%) (IU/dL) | Dosing Frequency (hours) / Duration of Treatment (days) |
Hemorrhage | ||
Early hemarthrosis, muscle hemorrhage, or oral hemorrhage | 20–40 | Repeat every 12 to 24 hours. For at least 1 day until the hemorrhagic episode, as indicated by pain, is resolved or healing is achieved. |
More extensive hemarthrosis, muscle hemorrhage, or hematoma | 30-60 | Repeat infusion every 12 to 24 hours for 3 to 4 days or more, until pain and acute disability cease. |
Potentially life-threatening hemorrhage | 60-100 | Repeat infusion every 8 to 24 hours until the danger has passed |
Surgery | ||
Minor surgery, including dental extraction | 30-60 | Every 24 hours, for at least 1 day, until healing is achieved. |
Major surgery | 80-100 (pre- and post-operative) | Repeat infusion every 8-24 hours until adequate wound healing is achieved, and then for at least another 7 days of treatment to maintain a factor VIII activity of 30% to 60% (IU/dL). |
INSTRUCTIONS FOR PREPARATION AND ADMINISTRATION
- Allow the solvent syringe (water for injections) and the powder to reach room temperature in the closed vial. You can do this by holding them in your hands until they reach the same temperature as your hands. Do not heat the vial and pre-filled syringe in any other way. This temperature should be maintained during reconstitution.
- Remove the flip-off plastic cap from the powder vial to expose the central part of the rubber stopper. Do not remove the gray stopper or the metal ring surrounding the top of the vial.
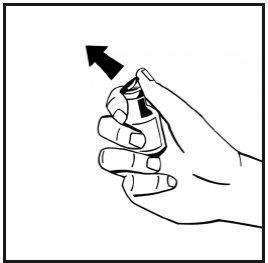
- Clean the top of the vial with an alcohol swab. Let the alcohol dry.
- Remove the paper cover from the vial adapter package. Do not remove the adapter from its package.
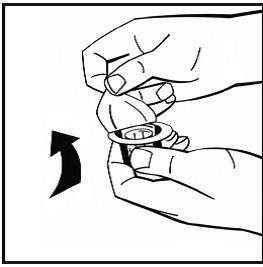
- Place the powder vial on a flat surface and hold it. Take the adapter package and place the vial adapter over the center of the rubber stopper of the powder vial. Press the adapter package firmly downwards until the adapter spike penetrates the rubber stopper. The adapter will be secured to the vial when done.
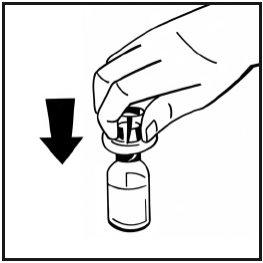
- Remove the paper cover from the pre-filled syringe package. Hold the plunger rod by the end and do not touch the shaft. Attach the threaded end of the plunger rod to the syringe plunger. Turn the plunger rod clockwise until you feel a slight resistance.
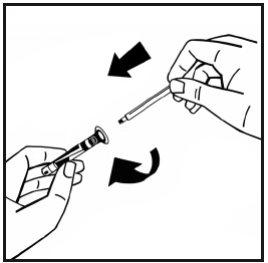
- Break the plastic protective tip of the solvent syringe by snapping the perforation of the flip-off cap. Do not touch the inside of the flip-off cap or the tip of the syringe. If you do not use the solution immediately, close the filled syringe with the plastic protective tip for storage.
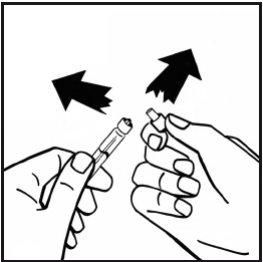
- Remove the adapter packaging and discard it.
- Firmly attach the solvent syringe to the vial adapter by turning it clockwise until you feel a slight resistance.
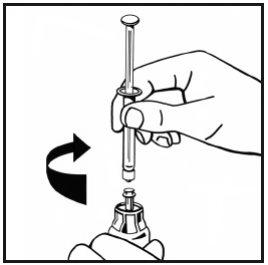
- Slowly inject all of the solvent into the powder vial by pressing the plunger rod downwards.
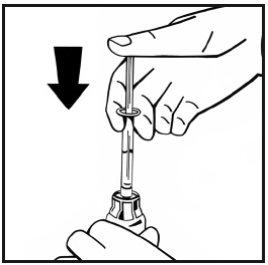
- Without removing the syringe, gently swirl or rotate the vial a few times to dissolve the powder. Do not shake. Wait until all of the powder is completely dissolved.
- Check the final solution for particles before administering. The solution should be clear and colorless, practically free from visible particles. Do not use cloudy or sedimented solutions.
- Turn the vial attached to the syringe upside down, and slowly withdraw the solution into the syringe. Make sure to transfer all of the vial contents into the syringe.
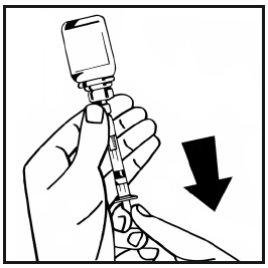
- Separate the filled syringe from the vial adapter by turning it counterclockwise and discard the empty vial.
- The solution is now ready for immediate use. Do not refrigerate.
- Clean the chosen injection site with one of the provided alcohol swabs.
- Attach the provided injection kit to the syringe. Insert the injection kit needle into the chosen vein. If you have used a tourniquet to make the vein more visible, it should be released before starting to inject the solution. Blood should not enter the syringe due to the risk of fibrin clot formation.
- Inject the solution into the vein slowly, no faster than 4 mL per minute.
If you use more than one vial of powder for a treatment, you can use the same needle again. The vial adapter and syringe are for single use only.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NUWIQ 1500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 1,000 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1500 IUActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription requiredDosage form: INJECTABLE, 1000 IU - after reconstitution in 2 ml of water for injections, the dose is 500 IU/mlActive substance: coagulation factor VIIIManufacturer: Takeda Manufacturing Austria AgPrescription required
Online doctors for NUWIQ 1500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about NUWIQ 1500 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















