AMGEVITA 40 mg SOLUTION FOR INJECTION IN PRE-FILLED PEN

How to use AMGEVITA 40 mg SOLUTION FOR INJECTION IN PRE-FILLED PEN
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
AMGEVITA 40mg solution for injection in pre-filled pen
adalimumab
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- Your doctor will give you a patient information card, which contains important safety information that you need to know before and during treatment with AMGEVITA. Keep this patient information card.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is AMGEVITA and what is it used for
- What you need to know before you use AMGEVITA
- How to use AMGEVITA
- Possible side effects
- Storing AMGEVITA
- Contents of the pack and other information
1. What is AMGEVITA and what is it used for
AMGEVITA contains the active substance adalimumab, a medicine that acts on the body's immune system (defense).
AMGEVITA is indicated for the treatment of inflammatory diseases described below:
- Rheumatoid arthritis
- Juvenile idiopathic polyarticular arthritis
- Enthesitis-related arthritis
- Ankylosing spondylitis
- Axial spondyloarthritis without radiographic evidence of ankylosing spondylitis
- Psoriatic arthritis
- Plaque psoriasis
- Hidradenitis suppurativa
- Crohn's disease
- Ulcerative colitis
- Non-infectious uveitis
The active substance in AMGEVITA, adalimumab, is a human monoclonal antibody. Monoclonal antibodies are proteins that target a specific target.
The target of adalimumab is a protein called tumor necrosis factor (TNFα), which is involved in the immune system (defense) and is found in high levels in the inflammatory diseases described above. By targeting TNFα, AMGEVITA reduces the inflammation process in these diseases.
Rheumatoid arthritis
Rheumatoid arthritis is an inflammatory disease of the joints.
AMGEVITA is used to treat rheumatoid arthritis in adults. If you have active moderate to severe rheumatoid arthritis, you may have been given other medicines that modify the disease, such as methotrexate, beforehand. If you do not respond well to these medicines, you will be given AMGEVITA to treat your rheumatoid arthritis.
AMGEVITA can also be used to treat severe, active, and progressive rheumatoid arthritis without prior treatment with methotrexate.
AMGEVITA reduces the damage to the cartilage and bones of the joints caused by the disease and improves physical function.
AMGEVITA is usually used in combination with methotrexate. If your doctor decides that methotrexate is not suitable for you, AMGEVITA can be given alone.
Juvenile idiopathic polyarticular arthritis and enthesitis-related arthritis
Juvenile idiopathic polyarticular arthritis and enthesitis-related arthritis are inflammatory diseases of the joints that usually appear for the first time in childhood.
AMGEVITA is used to treat juvenile idiopathic polyarticular arthritis in patients from 2 years of age and enthesitis-related arthritis in patients from 6 years of age. You may have received other disease-modifying medicines, such as methotrexate, beforehand. If you do not respond well to these medicines, you will be given AMGEVITA to treat your juvenile idiopathic polyarticular arthritis or enthesitis-related arthritis.
Ankylosing spondylitis and axial spondyloarthritis without radiographic evidence of ankylosing spondylitis
Ankylosing spondylitis and axial spondyloarthritis without radiographic evidence of ankylosing spondylitis are inflammatory diseases that affect the spine.
AMGEVITA is used to treat ankylosing spondylitis and axial spondyloarthritis without radiographic evidence of ankylosing spondylitis in adults. If you have ankylosing spondylitis or axial spondyloarthritis without radiographic evidence of ankylosing spondylitis, you will be treated first with other medicines, and if you do not respond well to these medicines, you will be given AMGEVITA to reduce the signs and symptoms of your disease.
Psoriatic arthritis
Psoriatic arthritis is an inflammation of the joints associated with psoriasis.
AMGEVITA is used to treat psoriatic arthritis in adults. AMGEVITA reduces the joint damage caused by the disease in the cartilage and bone and improves physical function.
Plaque psoriasis in adults and children
Plaque psoriasis is a skin disease that causes red, scaly, crusty, and silvery-scaled patches. Plaque psoriasis can also affect the nails, causing them to deteriorate, thicken, and lift off the nail bed, which can be painful. It is believed that psoriasis is caused by a defect in the body's immune system that leads to an increase in skin cell production.
AMGEVITA is used to treat moderate to severe plaque psoriasis in adults. AMGEVITA is also used to treat severe plaque psoriasis in children and adolescents between 4 and 17 years of age who have not responded or are not suitable for topical treatments and phototherapies.
Hidradenitis suppurativa in adults and adolescents
Hidradenitis suppurativa (also known as inverse acne) is a chronic and often painful inflammatory skin disease. Symptoms can include sensitive nodules (lumps) and abscesses (boils) that can discharge pus. It usually affects specific areas of the skin, such as under the breast, armpits, inner thighs, groin, and buttocks. There may also be scarring in the affected areas.
AMGEVITA is used to treat hidradenitis suppurativa in adults and adolescents from 12 years of age. AMGEVITA can reduce the number of nodules and abscesses and the pain that is usually associated with this disease. You may have received other medicines beforehand. If you do not respond well to these medicines, you will be given AMGEVITA.
Crohn's disease in adults and children
Crohn's disease is an inflammatory disease of the digestive tract.
AMGEVITA is used to treat Crohn's disease in adults and children between 6 and 17 years of age. If you have Crohn's disease, you will be treated first with other medicines, and if you do not respond well to these medicines, you will be given AMGEVITA to reduce the signs and symptoms of Crohn's disease.
Ulcerative colitis in adults and children
Ulcerative colitis is an inflammatory disease of the large intestine.
AMGEVITA is used to treat moderate to severe ulcerative colitis in adults and children between 6 and 17 years of age. If you have ulcerative colitis, you may be given other medicines first. If you do not respond well to these medicines, you will be given AMGEVITA to reduce the signs and symptoms of your disease.
Non-infectious uveitis in adults and children
Non-infectious uveitis is an inflammatory disease that affects certain parts of the eye.
AMGEVITA is used to treat
- Adults with non-infectious uveitis with inflammation that affects the back of the eye.
- Children from 2 years of age with chronic non-infectious uveitis with inflammation that affects the front of the eye.
This inflammation can lead to a decrease in vision and/or the presence of floaters in the eye (black dots or thin lines that move across the field of vision). AMGEVITA works by reducing this inflammation.
2. What you need to know before you use AMGEVITA
Do not use AMGEVITA
- if you are allergic to adalimumab or any of the other ingredients of this medicine (listed in section 6).
- if you have a severe infection, including active tuberculosis, sepsis (blood infection), or other opportunistic infections (unusual infections associated with a weakened immune system (see "Warnings and precautions"). If you have symptoms of any infection, such as fever, wounds, fatigue, dental problems, it is important that you inform your doctor.
- if you have moderate to severe heart failure. It is important that you tell your doctor if you have had or have any serious heart problems (see "Warnings and precautions").
Warnings and precautions
Talk to your doctor or pharmacist before you start using AMGEVITA:
Allergic reactions
- If you notice an allergic reaction with symptoms such as chest tightness, difficulty breathing, dizziness, swelling, or rash, stop using AMGEVITA and contact your doctor immediately, as these reactions can be life-threatening.
Infections
- If you have any infection, including chronic or localized infections (such as a leg ulcer), talk to your doctor before starting treatment with AMGEVITA. If you are not sure, contact your doctor.
- With AMGEVITA treatment, you may be more likely to get infections. This risk may be greater if you have damaged lungs. These infections can be serious and include tuberculosis, viral, fungal, parasitic, or bacterial infections, or other opportunistic infections and sepsis that can, in rare cases, be life-threatening. For this reason, it is important that if you have symptoms such as fever, wounds, fatigue, or dental problems, you tell your doctor. Your doctor may recommend that you temporarily stop using AMGEVITA.
Tuberculosis
- Since cases of tuberculosis have been reported in patients treated with adalimumab, your doctor will examine you for signs or symptoms of tuberculosis before starting your treatment with AMGEVITA. This will include a thorough medical examination, including your medical history and appropriate diagnostic tests (such as chest X-ray and tuberculin test). The results of these tests should be recorded on your patient information card. It is very important that you inform your doctor if you have had tuberculosis or have been in contact with a patient with tuberculosis.
- Tuberculosis can develop during treatment, even if you have received preventive treatment for tuberculosis.
- If symptoms of tuberculosis (persistent cough, weight loss, general malaise, low-grade fever) or any other infection appear during or after treatment, contact your doctor immediately.
Recurrent/infectious diseases
- Tell your doctor if you live or travel in areas where fungal infections such as histoplasmosis, coccidioidomycosis, or blastomycosis are common.
- Tell your doctor if you have a history of recurrent infections or other conditions or factors that increase the risk of infections.
Hepatitis B virus
- Tell your doctor if you are a carrier of the hepatitis B virus (HBV), if you have had active HBV infections, or if you think you may be at risk of getting HBV. Your doctor should perform an HBV test. AMGEVITA can cause the reactivation of HBV in people who carry this virus. In rare cases, especially if you are taking other medicines that suppress the immune system, the reactivation of HBV can be life-threatening.
Being over 65 years old
- If you are over 65 years old, you may be more likely to get infections while being treated with AMGEVITA. You and your doctor should pay special attention to the appearance of signs of infection while you are being treated with AMGEVITA. It is important that you inform your doctor if you have symptoms of infections, such as fever, wounds, fatigue, or dental problems.
Surgical or dental interventions
- If you are going to have surgery or dental treatment, tell your doctor that you are taking AMGEVITA. Your doctor may recommend that you temporarily stop using AMGEVITA.
Demyelinating disease
- If you have or develop a demyelinating disease such as multiple sclerosis, your doctor will decide whether you should be treated or continue treatment with AMGEVITA. Tell your doctor immediately if you experience symptoms such as changes in vision, weakness in arms or legs, or numbness or tingling in any part of your body.
Vaccines
- Certain vaccines contain live, attenuated bacteria or viruses that can cause infections and should not be given if you are being treated with AMGEVITA. Talk to your doctor before receiving any vaccine. If possible, it is recommended that children update their vaccination schedule according to current vaccination guidelines before starting treatment with AMGEVITA.
- If you receive AMGEVITA while pregnant, your child may have a higher risk of getting infections during the 5 months following the last dose of AMGEVITA you received during pregnancy. It is important that you inform your child's doctor and other healthcare professionals about your use of AMGEVITA during pregnancy, so they can decide whether your child should receive any vaccine.
Heart failure
- If you have mild heart failure and are being treated with AMGEVITA, your doctor should monitor your heart failure closely. It is important that you inform your doctor if you have had or have any serious heart problems. If new symptoms of heart failure appear or existing ones worsen (such as difficulty breathing or swelling of the feet), you should contact your doctor immediately. Your doctor will decide whether you should continue using AMGEVITA.
Fever, bruising, bleeding, or pallor
- In some patients, the body may be unable to produce enough of the type of blood cells that help the body fight infections (white blood cells) or those that help stop bleeding (platelets). If you have persistent fever, bruising, or bleeding easily, or are very pale, talk to your doctor immediately. Your doctor may decide to interrupt treatment.
Cancer
- In very rare cases, certain types of cancer have been reported in children and adults treated with adalimumab or other TNF-blocking agents. People with rheumatoid arthritis with more severe and longer-lasting disease may have a higher than average risk of developing lymphoma (a cancer that affects the lymphatic system) and leukemia (a cancer that affects the blood and bone marrow).
- If you are being treated with AMGEVITA, the risk of getting lymphoma, leukemia, and other types of cancer may increase. A specific and severe type of lymphoma has been observed in rare cases in patients treated with adalimumab. Some of these patients were also receiving treatment with azathioprine or 6-mercaptopurine. Tell your doctor if you are taking azathioprine or 6-mercaptopurine with AMGEVITA.
- Additionally, cases of non-melanoma skin cancer have been observed in patients using adalimumab. Tell your doctor if new skin lesions appear or existing ones change during or after treatment.
- Cancers other than lymphoma have been reported in patients with a certain lung disease, called Chronic Obstructive Pulmonary Disease (COPD), treated with another TNF-blocking agent. If you have COPD or are a heavy smoker, you should talk to your doctor about whether treatment with a TNF blocker is suitable for you.
Autoimmune diseases
- In rare cases, treatment with AMGEVITA may lead to a lupus-like syndrome. Contact your doctor if you have symptoms such as unexplained persistent rash, fever, joint pain, or fatigue.
In order to improve the traceability of this medicine, your doctor or pharmacist should record the name and batch number of the medicine administered in your medical history. If you are asked for this information in the future, you can also take note of these details.
Children and adolescents
- Vaccines: if possible, your child should be up-to-date with all vaccinations before using AMGEVITA.
- Do not give AMGEVITA to children with juvenile idiopathic polyarticular arthritis under 2 years of age.
- Do not give AMGEVITA to children with plaque psoriasis under 4 years of age.
- Do not give AMGEVITA to children with Crohn's disease or ulcerative colitis under 6 years of age.
Using AMGEVITA with other medicines
Tell your doctor or pharmacist if you are taking or have recently taken or might take any other medicines.
AMGEVITA can be taken with methotrexate or with certain disease-modifying antirheumatic drugs (sulfasalazine, hydroxychloroquine, leflunomide, and injectable gold salts), steroids, or painkillers, including non-steroidal anti-inflammatory drugs (NSAIDs).
Do not use AMGEVITA with medicines whose active substances are anakinra or abatacept due to an increased risk of serious infections. If you are unsure, talk to your doctor.
Pregnancy and Breastfeeding
- You should consider using appropriate contraceptive methods to avoid becoming pregnant and continue using them for at least 5 months after the last treatment with AMGEVITA.
- If you are pregnant, think you may be pregnant, or plan to have a baby, ask your doctor for advice on using this medicine.
- AMGEVITA should be used during pregnancy only if necessary.
- According to a pregnancy study, there was no increased risk of congenital defects when the mother had received treatment with AMGEVITA during pregnancy compared to mothers with the same disease who did not receive treatment with AMGEVITA.
- AMGEVITA can be used during breastfeeding.
- If you use AMGEVITA while pregnant, your child may have a higher risk of contracting an infection.
- It is essential that you inform the pediatrician and other healthcare professionals about the use of AMGEVITA during pregnancy before the baby receives any vaccine. For more information on vaccines, see the "Warnings and Precautions" section.
Driving and Using Machines
The influence of AMGEVITA on the ability to drive, ride a bicycle, or use machines is small. You may experience dizziness (vertigo) and vision disturbances after taking AMGEVITA.
AMGEVITA contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per 0.8 ml dose; it is essentially "sodium-free".
3. How to Use AMGEVITA
Follow exactly the administration instructions for this medicine indicated by your doctor. In case of doubt, consult your doctor again.
Adults with Rheumatoid Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis, or Axial Spondyloarthritis without Radiographic Evidence of Ankylosing Spondylitis
AMGEVITA is injected under the skin (subcutaneously). The normal dose in adults with rheumatoid arthritis, ankylosing spondylitis, axial spondyloarthritis without radiographic evidence of ankylosing spondylitis, and for patients with psoriatic arthritis is 40 mg administered every other week as a single dose.
In the case of rheumatoid arthritis, treatment with methotrexate is maintained during the use of AMGEVITA. If your doctor determines that methotrexate is inappropriate, AMGEVITA can be administered alone.
If you have rheumatoid arthritis and do not receive methotrexate during your treatment with AMGEVITA, your doctor may decide to give you 40 mg every week or 80 mg every two weeks.
Children, Adolescents, and Adults with Juvenile Idiopathic Polyarticular Arthritis
Children, adolescents, and adults from 2 years of age with a weight of 30 kg or more
The recommended dose of AMGEVITA is 40 mg administered every other week.
Children, Adolescents, and Adults with Enthesitis-Related Arthritis
Children, adolescents, and adults from 6 years of age with a weight of 30 kg or more
The recommended dose of AMGEVITA is 40 mg every other week.
Adults with Plaque Psoriasis
The usual dosage in adults with plaque psoriasis consists of an initial dose of 80 mg (as two 40 mg injections on one day), followed by 40 mg every other week starting one week after the initial dose. You should continue injecting AMGEVITA for as long as your doctor has indicated. Depending on your response, your doctor may increase the dose to 40 mg weekly or 80 mg every two weeks.
Children and Adolescents with Plaque Psoriasis
Children and adolescents from 4 to 17 years of age with a weight of 30 kg or more
The recommended dose of AMGEVITA is an initial dose of 40 mg, followed by 40 mg one week later. Then, the usual dose is 40 mg every other week.
Adults with Hidradenitis Suppurativa
The usual dosing regimen for hidradenitis suppurativa is an initial dose of 160 mg (as four 40 mg injections on one day or two 40 mg injections per day for two consecutive days), followed by a dose of 80 mg (as two 40 mg injections on one day) two weeks later. After two more weeks, continue with a dose of 40 mg weekly or 80 mg every two weeks, as prescribed by your doctor. It is recommended that you use an antiseptic liquid daily on the affected areas.
Adolescents with Hidradenitis Suppurativa from 12 to 17 years of age, with a weight of 30 kg or more
The recommended dose of AMGEVITA is an initial dose of 80 mg (as two 40 mg injections on one day), followed by 40 mg every other week starting one week later. If you have an inadequate response to AMGEVITA 40 mg every other week, your doctor may increase the dose to 40 mg weekly or 80 mg every two weeks.
It is recommended that you use an antiseptic liquid daily on the affected areas.
Adults with Crohn's Disease
The usual dosing regimen for Crohn's disease is 80 mg (as two 40 mg injections on one day) initially, followed by 40 mg every other week starting two weeks later. If a faster response is required, your doctor may prescribe an initial dose of 160 mg (as four 40 mg injections on one day or two 40 mg injections per day for two consecutive days), followed by 80 mg (as two 40 mg injections on one day) two weeks later, and then 40 mg every other week. Depending on your response, your doctor may increase the dose to 40 mg weekly or 80 mg every two weeks.
Children and Adolescents with Crohn's Disease
Children and adolescents from 6 to 17 years of age with a weight less than 40 kg
The usual dosing regimen is 40 mg initially, followed by 20 mg two weeks later. If a faster response is required, your doctor may prescribe an initial dose of 80 mg (as two 40 mg injections on one day) followed by 40 mg two weeks later.
Then, the usual dose is 20 mg every other week. Depending on your response, your doctor may increase the frequency of the dose to 20 mg weekly.
The 40 mg pre-filled pen cannot be used for the 20 mg dose. A 20 mg pre-filled syringe is available for the 20 mg dose.
Children and adolescents from 6 to 17 years of age with a weight of 40 kg or more
The usual dosing regimen is 80 mg (as two 40 mg injections on one day) initially, followed by 40 mg two weeks later. If a faster response is required, your doctor may prescribe an initial dose of 160 mg (as four 40 mg injections on one day or two 40 mg injections per day for two consecutive days) followed by 80 mg (as two 40 mg injections on one day) two weeks later.
Then, the usual dose is 40 mg every other week. Depending on your response, your doctor may increase the dose to 40 mg weekly or 80 mg every two weeks.
Adults with Ulcerative Colitis
The usual dosage of AMGEVITA in adults with ulcerative colitis is 160 mg initially (as four 40 mg injections on one day or two 40 mg injections per day for two consecutive days), followed by 80 mg (as two 40 mg injections on one day) two weeks later, and then 40 mg every other week. Depending on your response, your doctor may increase the dose to 40 mg weekly or 80 mg every two weeks.
Children and Adolescents with Ulcerative Colitis
Children and adolescents from 6 years of age with a weight less than 40 kg
The usual dose of AMGEVITA is 80 mg (as two 40 mg injections on one day) initially, followed by a dose of 40 mg (as one 40 mg injection) two weeks later. Then, the usual dose is 40 mg every other week.
Patients who turn 18 years old while receiving treatment with 40 mg every other week should continue with their prescribed dose.
Children and adolescents from 6 years of age with a weight of 40 kg or more
The usual dose of AMGEVITA is 160 mg (as four 40 mg injections on one day or two 40 mg injections per day for two consecutive days) initially, followed by a dose of 80 mg (as two 40 mg injections on one day) two weeks later. Then, the usual dose is 80 mg every other week.
Patients who turn 18 years old while receiving treatment with 80 mg every other week should continue with their prescribed dose.
Adults with Non-Infectious Uveitis
The usual dose in adults with non-infectious uveitis is an initial dose of 80 mg (as two 40 mg injections on one day), followed by 40 mg every other week starting one week after the initial dose. You should continue injecting AMGEVITA for as long as your doctor has indicated.
In non-infectious uveitis, treatment with corticosteroids or other medicines that affect the immune system can be continued during the use of AMGEVITA. AMGEVITA can also be administered alone.
Children and Adolescents from 2 Years of Age with Chronic Non-Infectious Uveitis
Children and adolescents from 2 years of age with a weight less than 30 kg
The usual dose of AMGEVITA is 20 mg every other week along with methotrexate.
Your doctor may prescribe an initial dose of 40 mg that can be administered one week before starting the usual regimen.
The 40 mg pre-filled pen cannot be used for the 20 mg dose. A 20 mg pre-filled syringe is available for the 20 mg dose.
Children and adolescents from 2 years of age with a weight of 30 kg or more
The usual dose of AMGEVITA is 40 mg every other week along with methotrexate.
Your doctor may prescribe an initial dose of 80 mg that can be administered one week before starting the usual regimen.
Form and Route of Administration
AMGEVITA is injected under the skin (subcutaneously).
Detailed instructions on how to inject AMGEVITA are provided in the "Instructions for Use" section.
If You Use More AMGEVITA Than You Should
If you accidentally inject AMGEVITA more frequently than prescribed by your doctor, inform your doctor. Always carry the medicine box with you, even if it is empty.
If You Miss a Dose of AMGEVITA
If you forget to administer an injection, you should inject the next dose of AMGEVITA as soon as you remember. Then, the next dose will be administered as usual, as if you had not missed a dose.
If You Stop Treatment with AMGEVITA
The decision to stop using AMGEVITA should be discussed with your doctor. Your symptoms may return after stopping treatment.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them. Most adverse effects are mild to moderate. However, some can be serious and require treatment. Adverse effects may appear at least up to 4 months after the last injection of AMGEVITA.
Contact your doctor immediately if you notice any of the following signs of allergic reaction or heart failure:
- severe rash, hives, or other signs of allergic reaction;
- swelling of the face, hands, feet;
- difficulty breathing, swallowing;
- shortness of breath when exercising or lying down, swelling of feet.
Contact your doctor as soon as possible if you notice any of the following effects:
- signs of infection such as fever, nausea, wounds, dental problems, burning sensation when urinating;
- feeling of weakness or fatigue;
- cough;
- tingling;
- numbness;
- double vision;
- weakness in arms or legs;
- signs of skin cancer such as a lump or an open sore that does not heal;
- signs and symptoms of blood disorders such as persistent fever, bruising, bleeding, and paleness.
The symptoms described above may be signs of the adverse effects listed below, which have been observed with adalimumab.
Very common (may affect more than 1 in 10 people)
- reactions at the injection site (including pain, swelling, redness, or itching);
- respiratory tract infections (including colds, runny nose, sinusitis, pneumonia);
- headache;
- abdominal pain;
- nausea and vomiting;
- rash;
- muscle pain.
Common (may affect up to 1 in 10 people)
- severe infections (including sepsis and flu);
- intestinal infections (including gastroenteritis);
- skin infections (including cellulitis and herpes);
- ear infection;
- oral infections (including dental infection and mouth ulcers);
- infections of the reproductive system;
- urinary tract infection;
- fungal infections;
- joint infections;
- benign tumors;
- skin cancer;
- allergic reactions (including seasonal allergies);
- dehydration;
- mood changes (including depression);
- anxiety;
- difficulty sleeping;
- sensory disturbances such as tingling, itching, or numbness;
- migraine;
- nerve root compression (including lower back pain and leg pain);
- visual disturbances;
- eye inflammation;
- eyelid inflammation and eye swelling;
- vertigo (feeling of dizziness or as if the room is spinning);
- feeling of rapid heartbeat;
- high blood pressure;
- flushing;
- bruising;
- cough;
- asthma;
- difficulty breathing;
- gastrointestinal bleeding;
- dyspepsia (indigestion, bloating, and heartburn);
- acid reflux;
- dry eye syndrome (including dryness in eyes and mouth);
- itching;
- pruritic rash;
- bruising;
- skin inflammation (such as eczema);
- breaking of fingernails and toenails;
- increased sweating;
- hair loss;
- new or worsening psoriasis;
- muscle spasms;
- blood in urine;
- kidney problems;
- chest pain;
- edema;
- fever;
- low platelet count in blood, which increases the risk of bleeding or bruising;
- wound healing problems.
Uncommon (may affect up to 1 in 100 people)
- opportunistic infections (including tuberculosis and other infections that occur when disease resistance decreases);
- neurological infections (including viral meningitis);
- eye infections;
- bacterial infections;
- diverticulitis (inflammation and infection of the large intestine);
- cancer, including cancer that affects the lymphatic system (lymphoma) and melanoma (skin cancer);
- immune system disorders that can affect the lungs, skin, and lymph nodes (the most common presentation is sarcoidosis);
- vasculitis (inflammation of blood vessels);
- tremor;
- neuropathy;
- stroke;
- hearing loss, tinnitus;
- feeling of irregular heartbeat like skips;
- heart problems that can cause difficulty breathing or swelling of ankles;
- heart attack;
- aneurysm of a major artery, inflammation, and clotting in a vein, blockage of a blood vessel;
- pulmonary diseases that can cause difficulty breathing (including inflammation);
- pulmonary embolism (blockage of a pulmonary artery);
- pleural effusion (abnormal fluid accumulation in the pleural space);
- pancreatitis that causes severe abdominal and back pain;
- difficulty swallowing;
- facial edema;
- gallbladder inflammation; gallstones;
- fatty liver;
- night sweats;
- scars;
- abnormal muscle crisis;
- systemic lupus erythematosus (including skin inflammation, heart, lungs, joints, and other organs);
- sleep disturbances;
- impotence;
- inflammations.
Rare (may affect up to 1 in 1,000 people)
- leukemia (cancer that affects the blood and bone marrow);
- severe allergic reaction with shock;
- multiple sclerosis;
- nerve disorders (such as optic neuritis and Guillain-Barré syndrome that can cause muscle weakness, abnormal sensations, tingling in the arms and upper body);
- cardiac arrest;
- pulmonary fibrosis (scarring in the lung);
- intestinal perforation (hole in the intestinal wall);
- hepatitis (liver inflammation);
- reactivation of hepatitis B virus;
- autoimmune hepatitis (liver inflammation caused by the body's own immune system);
- cutaneous vasculitis (inflammation of blood vessels in the skin);
- Stevens-Johnson syndrome (potentially life-threatening reaction with flu-like symptoms and rash with blisters);
- facial edema associated with allergic reactions;
- erythema multiforme (inflammatory rash on the skin);
- lupus-like syndrome;
- angioedema (localized skin inflammation);
- lichenoid reaction on the skin (purple-red rash with itching).
Frequency not known (cannot be estimated from the available data)
- hepatosplenic T-cell lymphoma (rare and often fatal blood cancer);
- Merkel cell carcinoma (a type of skin cancer);
- Kaposi's sarcoma, a rare cancer related to human herpesvirus 8 infection. Kaposi's sarcoma usually manifests more frequently as purple-colored skin lesions;
- liver failure;
- worsening of a disease called dermatomyositis (seen as skin rash accompanied by muscle weakness);
- weight gain (for most patients, weight gain was reduced).
Some adverse effects observed with adalimumab do not have symptoms and can only be identified through a blood test. These include:
Very common (may affect more than 1 in 10 people)
- low white blood cell count;
- low red blood cell count;
- increased lipids in blood;
- increased liver enzymes.
Common (may affect up to 1 in 10 people)
- high white blood cell count;
- low platelet count;
- increased uric acid in blood;
- abnormal sodium levels in blood;
- low calcium level in blood;
- low phosphate level in blood;
- high blood sugar;
- high lactate dehydrogenase levels in blood;
- presence of autoantibodies in blood;
- low potassium level in blood.
Uncommon (may affect up to 1 in 100 people)
- elevated bilirubin levels (liver function test).
Rare (may affect up to 1 in 1,000 people)
- low counts in blood for white blood cells, red blood cells, and platelets.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that does not appear in this prospectus. You can also report them directly (see details below). By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
Spanish Medicines Surveillance System for Human Use:
www.notificaRAM.es
5. Storage of AMGEVITA
Keep this medicine out of sight and reach of children.
Do not use this medicine after the expiration date stated on the label and on the packaging after EXP or CAD. The expiration date is the last day of the month indicated.
Store in a refrigerator (between 2 °C and 8 °C). Do not freeze.
Store in the original packaging to protect it from light.
You can store a single AMGEVITA pre-filled pen at temperatures up to a maximum of 25 °C for a maximum period of 14 days. The pre-filled pen must be protected from light and must be discarded if not used within 14 days.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of AMGEVITA
- The active substance is adalimumab. Each pen contains 40 mg of adalimumab in 0.4 ml of solution.
- The other components are L-lactic acid, sucrose, polysorbate 80, sodium hydroxide, and water for injectable preparations.
Appearance and Package Contents of the Product
AMGEVITA is a clear, colorless to slightly yellowish solution.
Each package contains 1, 2, or 6 single-use pre-filled SureClick pens with 40 mg of adalimumab.
Marketing Authorization Holder and Manufacturer
Amgen Europe B.V.
Minervum 7061
4817 ZK Breda
Netherlands
Marketing Authorization Holder
Amgen Europe B.V.
Minervum 7061
4817 ZK Breda
Netherlands
Manufacturer
Amgen Technology Ireland UC
Pottery Road
Dun Laoghaire
Co Dublin
Ireland
Manufacturer
Amgen NV
Telecomlaan 5-7
1831 Diegem
Belgium
You can request more information about this medication by contacting the local representative of the marketing authorization holder.
Belgium/Belgique/Belgien s.a. Amgen n.v. Tel: +32 (0)2 7752711
Czech Republic Amgen s.r.o. Tel: +420 221 773 500 Denmark Amgen, filial af Amgen AB, Sverige Tlf: +45 39617500 Germany Amgen GmbH Tel: +49 89 1490960 Estonia Amgen Switzerland AG Vilniaus filialas Tel: +372 586 09553 Greece Amgen Ελλάς Φαρμακευτική Ε.Π.Ε. Τηλ: +30 210 3447000 Spain Amgen S.A. Tel: +34 93 600 18 60 France Amgen S.A.S. Tél: +33 (0)9 69 363 363 Croatia Amgen d.o.o. Tel: +385 (0)1 562 57 20 Ireland Amgen Ireland Limited Tel: +353 1 8527400 Iceland Vistor hf. Sími: +354 535 7000 Italy Amgen S.r.l. Tel: +39 02 6241121 Cyprus C.A. Papaellinas Ltd Τηλ: +357 22741 741 Latvia Amgen Switzerland AG Rigas filiale Tel: +371 257 25888 | Lithuania Amgen Switzerland AG Vilniaus filialas Tel: +370 5 219 7474 Luxembourg/Luxemburg s.a. Amgen Belgique/Belgien Tél/Tel: +32 (0)2 7752711 Hungary Amgen Kft. Tel.: +36 1 35 44 700 Malta Amgen S.r.l. Italy Tel: +39 02 6241121 Netherlands Amgen B.V. Tel: +31 (0)76 5732500 Norway Amgen AB Tlf: +47 23308000 Austria Amgen GmbH Tel: +43 (0)1 50 217 Poland Amgen Biotechnologia Sp. z o.o. Tel.: +48 22 581 3000 Portugal Amgen Biofarmacêutica, Lda. Tel: +351 21 4220606 Romania Amgen România SRL Tel: +4021 527 3000 Slovenia AMGEN zdravila d.o.o. Tel: +386 (0)1 585 1767 Slovakia Amgen Slovakia s.r.o. Tel: +421 2 321 114 49 Finland Orion Pharma Puh/Tel: +358 10 4261 Sweden Amgen AB Tel: +46 (0)8 6951100 United Kingdom (Northern Ireland)Amgen Limited Tel: +44 (0)1223 420305 |
Date of last revision of this leaflet: May 2024
Other sources of information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
INSTRUCTIONS FOR USE 40mg/0.4ml | ||||||||||||||
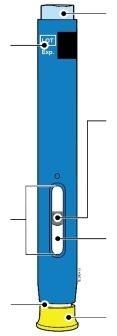
Get to know your SureClick pre-filled pen | ||||||||||||||
|
40 mg/0.4 ml |
|
1 | Important information you need to know before injecting AMGEVITA |
Using the AMGEVITA SureClick pre-filled pen: | |
It is important that you do not attempt to self-administer the injection until you have read and fully understood these instructions for use and have received training from your doctor or healthcare professional. | |
Do notuse the pre-filled pen if the carton is damaged or the seal is broken. | |
Do notuse the pre-filled pen after the expiry date shown on the label. | |
Do notshake the pre-filled pen. | |
Do notremove the yellow cap from the pre-filled pen until you are ready to inject. | |
Do notuse the pre-filled pen if it has been frozen. | |
Do notuse the pre-filled pen if it has been dropped onto a hard surface. Some parts of the pre-filled pen may be broken, even if you cannot see the break. Use a new pre-filled pen and contact your doctor or healthcare professional. | |
|
2 | Preparing for the injection of AMGEVITA |
2a | Wait for 30 minutes to allow the pre-filled pen to reach room temperature. |
WAIT 30 minutes | |
Take out the number of pre-filled pens you need for the injection and return any unused pre-filled pens to the refrigerator. | |
Let the pre-filled pen come to room temperature naturally. | |
Do notwarm it up with hot water, in a microwave, or by exposing it to direct sunlight. | |
Do notshake the pre-filled pen at any time. | |
Do notput the pre-filled pen back in the refrigerator once it has reached room temperature. | |
Using the pre-filled pen at room temperature allows for a more comfortable injection. |
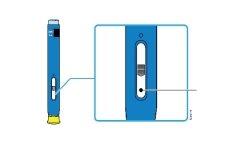
2b | Inspect the medicine. It should be clear and colorless or slightly yellowish. | |
| Medicine | |
It is normal for air bubbles to appear. | ||
Do notuse it if the medicine is cloudy, discolored, or contains particles. |
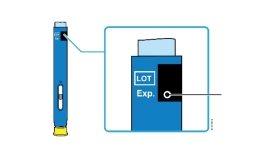
2c | Check the expiry date (EXP.) and inspect the pre-filled pen for any damage. | |
| Expiry date | |
Do notuse it if it has passed the expiry date. | ||
Do notuse the pre-filled pen if:
| ||
Make sure you have the correct medicine and dose. | ||
|
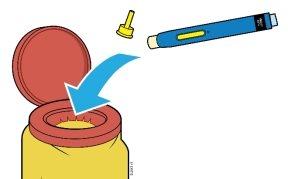
3 | Preparing for the injection | |
3a | Gather and place the materials for the injection on a clean and well-lit surface. | |
Sharps container |
| Alcohol swab Cotton ball or gauze |
AMGEVITA pre-filled pen (at room temperature) | ||
A sharps container | ||
Alcohol swabs | ||
A cotton ball or gauze |
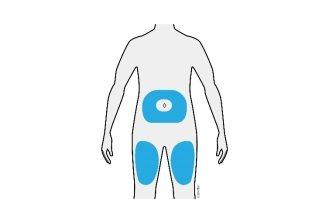
3b | Inject into one of these areas. |
| |
Inject into the thigh or abdomen (except for the area 5 centimeters around the navel). | |
Choose a different area for each injection. | |
Wash your hands with plenty of water and soap. | |
Clean the injection site with an alcohol swab. | |
Let the skin dry on its own. | |
Do nottouch this area again before the injection. | |
|
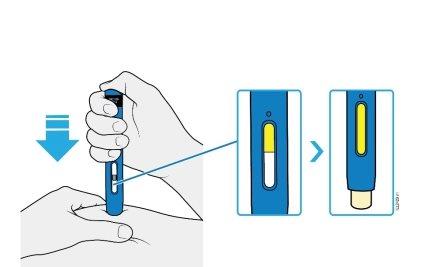
4 | Injecting AMGEVITA |
| |
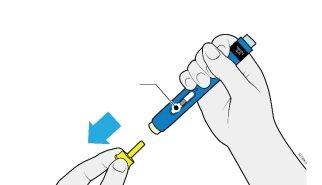
4a | Hold the pre-filled pen so that you can see the window. Remove the yellow cap. You may need to pull hard. |
The window should be visible |
|
Do nottwist, bend, or shake the yellow cap to remove it. | |
Do notput the needle cap back on, as this could damage the needle. | |
Do notput your finger into the cream-colored safety protector. | |
It is normal to see a drop of medicine at the end of the needle or in the cream-colored safety protector. |
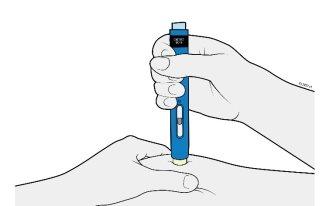
4b | Pinch the skin to create a firm surface at the injection site. Place the cream-colored safety protector straight onto the skin. |
PINCH | |
| |
Keep the skin pincheduntil the injection is complete. | |
Make sure you can see the window. | |
Make sure the autoinjector is placed straight onto the injection site (at a 90-degree angle). | |
PRESS and hold against the skin | |
| |
4c | Press firmly against the skin until the cream-colored safety protector stops moving. Hold the pen without lifting it. |
The cream-colored safety protector pushes and unlocks the light blue start button. |
PRESS the light blue start button | |
| |
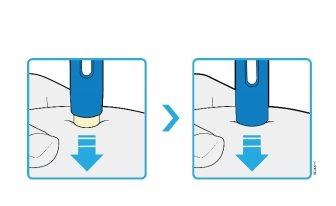
4d | Continue to press firmly against the skin and press the light blue start button to start the injection. |
You may hear or feel a click. | |
The window starts to turn yellow. | |
It is not a problem if you release the blue button. |
VISUAL CONFIRMATION the window will turn completely yellow | |
| |
4e | Continue to press against the skin. The window turns yellow when the injection is complete. |
The injection may take up to 10seconds to complete. | |
You may hear or feel a click. | |
Remove the pre-filled pen from the skin. | |
The cream-colored safety protector will lock around the needle. | |
|
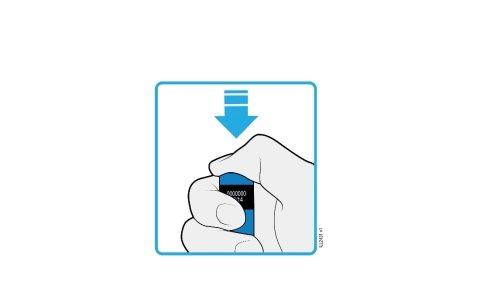
5 | Disposal and completion of AMGEVITA administration |
| |
| |
5a | Place the pre-filled pen and yellow cap in the sharps container. |
Do notreuse the pre-filled pen. | |
Do nottouch the cream-colored safety protector. | |
5b | Examine the injection site. |
Do notrub the injection site. | |
If you see blood, press the injection site with a cotton ball or gauze. Apply a bandage if necessary. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AMGEVITA 40 mg SOLUTION FOR INJECTION IN PRE-FILLED PENDosage form: INJECTABLE, 20 mgActive substance: adalimumabManufacturer: Amgen Europe B.V.Prescription requiredDosage form: INJECTABLE, 20 mgActive substance: adalimumabManufacturer: Amgen Europe B.V.Prescription requiredDosage form: INJECTABLE, 40 mgActive substance: adalimumabManufacturer: Amgen Europe B.V.Prescription required
Online doctors for AMGEVITA 40 mg SOLUTION FOR INJECTION IN PRE-FILLED PEN
Discuss questions about AMGEVITA 40 mg SOLUTION FOR INJECTION IN PRE-FILLED PEN, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions