VERRUPATCH 3,75 MG PARCHES CUTANEOS

Cómo usar VERRUPATCH 3,75 MG PARCHES CUTANEOS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Verrupatch 3,75mg parches cutáneos
ácido salicílico
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted. Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico o farmacéutico.
|
Contenido del prospecto
- Qué es Verrupatch y para qué se utiliza
- Qué necesita saber antes de empezar a usar Verrupatch
- Cómo usar Verrupatch
- Posibles efectos adversos
- Conservación de Verrupatch
- Contenido del envase e información adicional
1. Qué es Verrupatch y para qué se utiliza
Verrupatch es un medicamento en forma de parches cutáneos y pertenece al grupo de medicamentos antiverrugas.
Contiene como principio activo el ácido salicílico, con propiedades antiverrugas por su actividad queratolítica, que produce un ablandamiento y posterior destrucción del estrato córneo, permitiendo la eliminación de las verrugas.
Verrupatch está indicado en el tratamiento local de verrugas comunes, que suelen aparecer en las manos o en los pies (verrugas plantares).
2. Qué necesita saber antes de empezar a usar Verrupatch
No use Verrupatch
- Si es alérgico al acido salicílico o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- No aplicar sobre lunares, marcas de nacimiento, verrugas con pelos, verrugas genitales, verrugas de la cara o de las membranas mucosas.
- No utilizar este producto sobre piel irritada o herida ni sobre cualquier área que esté infectada, inflamada o enrojecida.
- No usar en niños menores de 2 años.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Verrupatch.
No ingerir. Uso cutáneo (exclusivamente sobre la piel).
Evitar el contacto con la piel sana.
Evitar el contacto con los ojos.
En caso de aplicación accidental en los ojos, enjuagarse con abundante agua.
Las personas diabéticas o con mala circulación deberían consultar a su médico antes de utilizar este medicamento.
Niños
No utilizar en niños entre 2 y 12 años sin el asesoramiento de un médico (Ver No use Verrupatch).
Los niños pequeños pueden tener mayor riesgo de efectos adversos.
Otros medicamentos y Verrupatch
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
No utilizar este medicamento junto con otros queratolíticos (antiverrugas) en la misma zona, ya que podrían potenciar su efecto.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No debe utilizar Verrupatch durante el embarazo, excepto para el tratamiento a corto plazo de una pequeña verruga.
Las formas orales (p. ej., comprimidos) de esta clase de medicamento pueden causar efectos adversos en el feto. Se desconoce si los mismos riesgos son aplicables a Verrupatch cuando se utiliza en verrugas.
Se debe decidir si es necesario interrumpir la lactancia o interrumpir el tratamiento, considerando el beneficio de la lactancia para el niño y el beneficio del tratamiento para la madre.
C
La influencia de Verrupatch sobre la capacidad para conducir y utilizar máquinas es nula o insignificante.
Verrupatch contiene propilenglicol (E-1520)
Este medicamento contiene 11,25 mg de propilenglicol en cada unidad de dosis (parche).
El propilenglicol puede provocar irritación en la piel.
3. Cómo usar Verrupatch
Siga exactamente las instrucciones de administración del medicamento contenidas en este prospecto o las indicadas por su médico o farmacéutico. En caso de duda, pregunte a su médico o farmacéutico.
Uso cutáneo (en la piel).
La dosis recomendada es un parche de Verrupatch una vez al día, preferiblemente antes de acostarse.
Uso en niños
No utilizar en niños menores de 2 años.
En niños menores de 12 años y mayores de 2 años, no utilizar sin el asesoramiento de un médico y evitar el uso prolongado en niños.
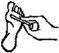
Instrucciones para la correcta administración

|
|
|
 Dejar actuar el parche durante la noche (unas 8 horas) y retirar por la mañana. Repetir este proceso diariamente, durante un máximo de 12 semanas, hasta la eliminación de la verruga. Generalmente se produce una mejoría apreciable durante los primeros días, por lo que se puede esperar una resolución completa tras un período de entre 3 y 6 semanas de tratamiento.
Dejar actuar el parche durante la noche (unas 8 horas) y retirar por la mañana. Repetir este proceso diariamente, durante un máximo de 12 semanas, hasta la eliminación de la verruga. Generalmente se produce una mejoría apreciable durante los primeros días, por lo que se puede esperar una resolución completa tras un período de entre 3 y 6 semanas de tratamiento.
Consulte a su médico o farmacéutico si no nota mejoría después de 1 semana, o si las verrugas son abundantes o si se produce infección o inflamación.
Si usa más Verrupatch del que debe
No son de esperar casos de sobredosificación dado el modo de aplicación de este medicamento. Un uso excesivo puede producir irritación, sobre todo en la piel sana. Utilizar, en su caso, emolientes.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame a un centro médico o consultar al Servicio de Información Toxicológica, Teléfono 91 562 04 20, indicando el medicamento y la cantidad usada.
Si olvidó usar Verrupatch
No aplique una dosis doble para compensar las dosis olvidadas. Aplique de nuevo el producto a su hora habitual
Si interrumpe el tratamiento con Verrupatch
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Puede producirse irritación local si los parches entran en contacto con la piel sana que rodea la verruga. En este caso, se recomienda suspender temporalmente el tratamiento hasta que la irritación desaparezca. Al reemprender el tratamiento, debe prestarse especial atención a que el parche esté adecuadamente recortado, para que sólo entre en contacto con el tejido de la verruga.
Se ha informado de la aparición de descamación excesiva en aplicación en lesiones abiertas; también reacciones alérgicas serias.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Verrupatch
Mantener este medicamento fuera de la vista y el alcance de los niños.
No requiere condiciones especiales de conservación.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
No utilice este medicamento si observa indicios visibles de deterioro en el aspecto de los parches cutáneos.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Verrupatch
- El principio activo es ácido salicílico. Cada parche contiene 3,75 mg de ácido salicílico.
- Los demás componentes (excipientes) son: propilenglicol (E-1520), goma de karaya, polietilenglicol 300 (Macrogol 300) y quaternium-15.
Aspecto del producto y contenido del envase
Verrupatch se presenta en forma de parches cutáneos circulares, de 6 mm de diámetro, de color beige y cubiertos por una lámina azul de polietileno que retiene la humedad, lo que ejerce un efecto oclusivo.
Cada envase contiene 20 parches dispuestos en dos sobres herméticos resistentes a la humedad, con 10 parches cada uno.
Para fijar los parches en la posición adecuada, se incorporan 20 tiras adhesivas dermocompatibles. También se proporciona una lima, para eliminar los restos encontrados de la superficie de la verruga.
Titular de la autorización de comercialización
Laboratorios Viñas, S.A.
Provenza, 386
08025 - Barcelona
España
Responsable de la fabricación
Laboratorios Viñas, S.A.
Torrente Vidalet, 29
08012- Barcelona
España
Fecha de la última revisión de este prospecto:Noviembre 2024
La información detallada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.es/
- País de registro
- Requiere recetaNo
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a VERRUPATCH 3,75 MG PARCHES CUTANEOSForma farmacéutica: INYECTABLE, 1 mlPrincipio activo: TralokinumabFabricante: Leo Pharma A/SRequiere recetaForma farmacéutica: INYECTABLE, 300 mgPrincipio activo: TralokinumabFabricante: Leo Pharma A/SRequiere recetaForma farmacéutica: CAPSULA, 10 mgPrincipio activo: AlitretinoinaFabricante: Industrial Farmaceutica Cantabria S.A.Requiere receta
Médicos online para VERRUPATCH 3,75 MG PARCHES CUTANEOS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de VERRUPATCH 3,75 MG PARCHES CUTANEOS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes