
ADTRALZA 150 mg SOLUCION INYECTABLE EN JERINGA PRECARGADA

Cómo usar ADTRALZA 150 mg SOLUCION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Adtralza150mg solución inyectable en jeringa precargada
tralokinumab
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Adtralza y para qué se utiliza
- Qué necesita saber antes de empezar a usar Adtralza
- Cómo usar Adtralza
- Posibles efectos adversos
- Conservación de Adtralza
- Contenido del envase e información adicional
1. Qué es Adtralza y para qué se utiliza
Adtralza contiene el principio activo tralokinumab.
Tralokinumab es un anticuerpo monoclonal (un tipo de proteína) que bloquea la acción de una proteína llamada IL‑13. La IL‑13 juega un papel clave en la aparición de los síntomas de la dermatitis atópica.
Adtralza se utiliza para tratar a pacientes adultos y adolescentes a partir de 12 años de edad con dermatitis atópica de moderada a grave, también conocida como eccema atópico. Adtralza se puede utilizar solo o en combinación con otros medicamentos para el eccema atópico que se aplican en la piel.
El uso de Adtralza para tratar la dermatitis atópica puede mejorar su eccema y reducir el picor y el dolor de la piel asociados.
2. Qué necesita saber antes de empezar a usar Adtralza
No use Adtralza
- si es alérgico al tralokinumab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Si cree que podría ser alérgico, o no está seguro, consulte a su médico, farmacéutico o enfermero antes de usar Adtralza.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar Adtralza.
Reacciones alérgicas
En muy raras ocasiones, los medicamentos pueden causar reacciones alérgicas (hipersensibilidad) y reacciones alérgicas graves llamadas anafilácticas. Mientras use Adtralza debe observar signos de estas reacciones (es decir, problemas respiratorios, hinchazón de la cara, boca y lengua, desmayo, mareo, sensación de mareo (debido a la presión arterial baja), habones, picor y erupción cutánea).
Deje de utilizar Adtralza y avise a su médico o busque ayuda médica de inmediato si nota cualquier signo de reacción alérgica. Dichos signos se indican al principio de la sección 4.
Infección parasitaria en los intestinos
Adtralza puede reducir su resistencia a las infecciones causadas por parásitos. Cualquier infección parasitaria, se debe tratar antes de comenzar el tratamiento con Adtralza. Consulte con su médico si tiene diarrea, gases, malestar estomacal, heces grasientas y deshidratación, lo que podrían ser signos de infección parasitaria. Si vive en una región donde estas infecciones son comunes o si viaja a esa región, consulte con su médico.
Problemas oculares
Consulte con su médico si tiene problemas oculares nuevos o que empeoren, incluidos dolor ocular o cambios en la visión.
Niños y adolescentes
Aún se desconocen la seguridad y los beneficios de Adtralza en niños menores de 12 años de edad, de modo que no administre este medicamento a esta población.
Otros medicamentos y Adtralza
Informe a su médico o farmacéutico
- Si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
- Si se le ha administrado recientemente o se le va a administrar alguna vacuna.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o usted tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento. Se desconocen los efectos de Adtralza en mujeres embarazadas; por lo tanto, es preferible evitar su uso durante el embarazo a menos que el médico le aconseje utilizarlo.
Si procede, usted y su médico deberán decidir si dará el pecho o utilizará Adtralza. No debe hacer ambas cosas a la vez.
Conducción y uso de máquinas
Es poco probable que Adtralza reduzca su capacidad para conducir y utilizar máquinas.
Adtralzacontiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis de 150 mg; esto es, esencialmente “exento de sodio”.
3. Cómo usar Adtralza
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico, farmacéutico o enfermero. En caso de duda, consulte de nuevo a su médico, farmacéutico o enfermero.
Cada jeringa precargada contiene 150 mg de tralokinumab.
Cantidad de Adtralza que debe administrarse y duración del tratamiento
Dosis recomendada en adultos y adolescentes con dermatitis atópica:
- Su médico decidirá la cantidad de Adtralza que necesita y la duración del tratamiento.
- La primera dosis recomendada es de 600 mg (cuatro inyecciones de 150 mg), seguida de 300 mg (dos inyecciones de 150 mg) administradas cada 2 semanas. Según cómo responda al tratamiento, el médico decidirá si usted se puede administrar una dosis cada 4 semanas.
Adtralza se administra por una inyección debajo de su piel (inyección subcutánea). Su médico o enfermero y usted pueden decidir si se puede inyectar Adtralza usted mismo.
Inyéctese Adtralza solo después de que su médico o enfermero le hayan enseñado a hacerlo correctamente. El cuidador también podrá inyectarle Adtralza tras haber recibido la formación adecuada.
No agite la jeringa.
Lea atentamente las “Instrucciones de uso” antes de inyectarse Adtralza.
Si usa más Adtralza del que debe
Si usa más medicamento del que debe o si se ha administrado la dosis demasiado pronto, consulte a su médico, farmacéutico o enfermero.
Si olvidó usar Adtralza
Si ha olvidado inyectarse una dosis en el momento oportuno, inyéctese Adtralza lo antes posible. Posteriormente, la siguiente dosis se deberá inyectar según la pauta establecida.
Si interrumpe el tratamiento con Adtralza
No interrumpa el tratamiento con Adtralza sin consultar primero a su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Adtralza puede causar efectos adversos graves, incluyendo reacciones alérgicas (hipersensibilidad) como las reacciones anafilácticas; los signos pueden incluir:
- problemas respiratorios
- hinchazón de la cara, boca y lengua
- desmayo, mareo, sensación de mareo (presión arterial baja)
- habones
- picor
- erupción cutánea
Deje de usar Adtralza y contacte a su médico o consiga ayuda médica inmediatamente si nota cualquier signo de una reacción alérgica.
Otros efectos adversos
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- infecciones del tracto respiratorio superior (es decir, resfriado común y garganta irritada)
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- enrojecimiento y picor de los ojos
- infección de los ojos
- reacciones en la zona de inyección (es decir, enrojecimiento, hinchazón)
Poco frecuentes(pueden afectar hasta a 1 de cada 100 personas)
- inflamación de los ojos que puede causar dolor ocular o disminución de la visión
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Adtralza
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en el envase después de EXP. La fecha de caducidad es el último día del mes que se indica.
Conservar en el embalaje original para protegerlo de la luz.
Conservar en nevera (entre 2 °C y 8 °C). No congelar.
Si es necesario, Adtralza puede conservarse a temperatura ambiente de hasta 25 °C en el envase exterior durante un plazo máximo de 14 días. No conservar a temperatura superior a 25 °C. Deseche Adtralza si no lo utiliza en un plazo máximo de 14 días de conservación a temperatura ambiente.
Si necesita sacar el envase de la nevera de manera permanente, escriba la fecha en que lo saca en el envase y utilice Adtralza en un plazo de 14 días. Adtralza no deberá refrigerarse de nuevo durante este periodo.
No utilice este medicamento si observa que está turbio, decolorado o contiene partículas.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su médico, farmacéutico o enfermero cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Adtralza
- El principio activo es tralokinumab.
- Cada jeringa precargada contiene 150 mg de tralokinumab en 1 ml de solución inyectable.
- Los demás componentes son acetato de sodio trihidrato (E262), ácido acético (E260), cloruro de sodio, polisorbato 80 (E433) y agua para preparaciones inyectables.
Aspecto de Adtralza y contenido del envase
Adtralza es una solución de transparente a opalescente, de incolora a amarillo pálido, suministrada en una jeringa precargada de vidrio con una protección de la aguja.
Adtralza está disponible en envases unitarios que contienen 2 jeringas precargadas o en envases múltiples que contienen 4 (2 envases de 2) o 12 (6 envases de 2) jeringas precargadas.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
LEO Pharma A/S
Industriparken 55
DK‑2750 Ballerup
Dinamarca
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien LEO Pharma N.V./S.A Tél/Tel: +32 3 740 7868 | Lietuva LEO Pharma A/S Tel: +45 44 94 58 88 |
???????? Borola Ltd Te?.: +359 2 9156 136 | Luxembourg/Luxemburg LEO Pharma N.V./S.A Tél/Tel: +32 3 740 7868 |
Ceská republika LEO Pharma s.r.o. Tel: +420 225 992 272 | Magyarország LEO Pharma Tel: +36 1 439 6132 |
Danmark LEO Pharma AB Tlf: +45 70 22 49 11 | Malta E.J. Busuttil Ltd Tel: +356 2144 7184 ext. 125 |
Deutschland LEO Pharma GmbH Tel: +49 6102 2010 | Nederland LEO Pharma B.V. Tel: +31 205104141 |
Eesti LEO Pharma A/S Tel: +45 44 94 58 88 | Norge LEO Pharma AS Tlf: +47 22514900 |
Ελλ?δα LEO Pharmaceutical Hellas S.A. Τηλ: +30 210 68 34322 | Österreich LEO Pharma GmbH Tel: +43 1 503 6979 |
España Laboratorios LEO Pharma, S.A. Tel: +34 93 221 3366 | Polska LEO Pharma Sp. z o.o. Tel.: +48 22 244 18 40 |
France Laboratoires LEO Tél: +33 1 3014 4000 | Portugal LEO Farmacêuticos Lda. Tel: +351 21 711 0760 |
Hrvatska Remedia d.o.o Tel: +385 1 3778 770 Ireland LEO Laboratories Ltd Tel: +353 (0) 1 490 8924 | România LEO Pharma Romania Tel: +40 213121963 Slovenija Medical Intertrade d.o.o. Tel: +386 1 2529113 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika LEO Pharma s.r.o. Tel: +421 2 5939 6236 |
Italia LEO Pharma S.p.A Tel: +39 06 52625500 | Suomi/Finland LEO Pharma Oy Puh/Tel: +358 20 721 8440 |
Κ?προς The Star Medicines Importers Co. Ltd. Τηλ: +357 2537 1056 | Sverige LEO Pharma AB Tel: +46 40 3522 00 |
Latvija LEO Pharma A/S Tel: +45 44 94 58 88 | United Kingdom (Northern Ireland) LEO Laboratories Ltd Tel: +44 (0) 1844 347333 |
Fecha de la última revisión de este prospecto: Octubre 2022
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
Las instrucciones de uso con información sobre cómo inyectar Adtralza están disponibles en la otra cara de este prospecto.
Instrucciones de uso
Adtralza
tralokinumab
Solución inyectable en jeringa precargada
Lea estas instrucciones antes de empezar a usar las jeringas precargadas de Adtralza y cada vez que adquiera un nuevo envase, ya que es posible que incluyan información nueva. También puede consultar con su profesional sanitario sobre su enfermedad o su tratamiento.
Conserve estas instrucciones de uso para poder consultarlas de nuevo si lo necesita.
Cada jeringa precargada contiene150mg de tralokinumab.
Las jeringas precargadas de Adtralza son de un solo uso.
INFORMACIÓN IMPORTANTE
Información importante que necesita saber antes de inyectar Adtralza
- Antes de inyectarse Adtralza por primera vez, su profesional sanitario le mostrará cómo preparar e inyectar Adtralza utilizando las jeringas precargadas.
- Nose inyecte Adtralza hasta que se le haya mostrado cómo inyectárselo correctamente.
- Hable con su profesional sanitario si tiene alguna pregunta sobre cómo inyectar Adtralza correctamente.
- Para recibir la dosis completa, debe administrarse2inyecciones deAdtralza(1conjunto de inyecciones). Se recomienda que utilice una zona de inyección diferente para cada nuevo conjunto de inyecciones.
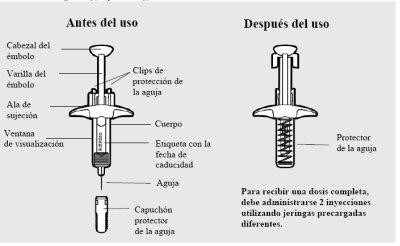
- Las jeringas precargadas de Adtralza tienen un protector de la aguja que cubrirá automáticamente la aguja al finalizar la inyección.
- Noretire el capuchón de la aguja hasta justo antes de administrar la inyección.
- Nocomparta ni reutilice las jeringas precargadas de Adtralza.
Partes de la jeringa precargada de Adtralza:
Conservación de Adtralza
- Mantenga Adtralza y todos los medicamentos fuera de la vista y del alcance de los niños.
- Conserve las jeringas precargadas de Adtralza en nevera entre 2 °C y 8 °C.
- Conserve las jeringas precargadas de Adtralza en el envase original para protegerlas de la luz hasta que esté listo para su uso.
- Nocongele las jeringas precargadas de Adtralza. Nolas utilice si se han congelado.
- Adtralza puede conservarse en el envase original a temperatura ambiente de hasta 25 °C durante un plazo máximo de 14 días. Si necesita sacar el envase de la nevera de manera permanente, escriba la fecha en que lo saca en el envase y utilice Adtralza en un plazo de 14 días. Deseche las jeringas si han estado fuera de la nevera durante más de 14 días.
Paso1: Preparación de la inyección de Adtralza
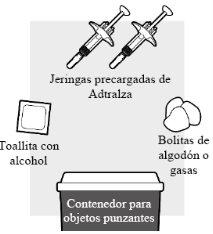
1a: Reúna todos los materiales necesarios para la inyección
Para cada dosis de Adtralza, necesitará:
- Una superficie de trabajo plana, limpia y bien iluminada, como una mesa
- Envase de Adtralza con 2 jeringas precargadas de Adtralza
- Una toallita con alcohol (no incluida en el envase)
- Gasas o bolitas de algodón limpias (no incluidas en el envase)
- Un contenedor para objetos punzantes para la eliminación de agujas (no incluido en el envase).
1b: Saque de la nevera el envase con la jeringa precargada de Adtralza
- Consulte la fecha de caducidad (EXP) que figura en el envase. Noutilice la jeringa si la fecha de caducidad ya ha pasado.
- Compruebe que el precinto del envase de Adtralza esté intacto. Noutilice las jeringas precargadas de Adtralza si el precinto del envase está roto.
No utilicelas jeringas precargadas de Adtralza que se hayan conservado a temperatura ambiente durante más de 14 días.

1c: Deje que las jeringas precargadas de Adtralza alcancen la temperatura ambiente
Deje el envase de Adtralza sobre una superficie plana y espere 30 minutos antes de inyectarse Adtralza, para dejar que las jeringas precargadas alcancen la temperatura ambiente (entre 20 °C y 25 °C). Esto ayudará a que la inyección de Adtralza sea más cómoda.
- Nocaliente las jeringas precargadas de ninguna manera.
- Noagite las jeringas.
- Noquite el capuchón de la aguja de las jeringas precargadas hasta que llegue al paso 3 y usted esté preparado para la inyección.
- Novuelva a guardar las jeringas en la nevera una vez que alcancen la temperatura ambiente.
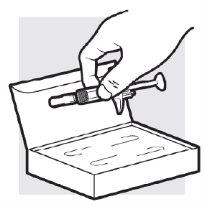
1d: Extraiga las jeringas precargadas de Adtralza del envase
Extraiga las 2jeringas precargadas de Adtralza del envase de una en una cogiéndolas por el medio del cuerpo (no la varilla del émbolo).
- Notoque los clips de protección de la aguja para evitar que se active el protector de la aguja demasiado pronto.
- Noquite el capuchón de la aguja de las jeringas precargadas hasta que llegue al paso 3 y usted esté preparado para la inyección.
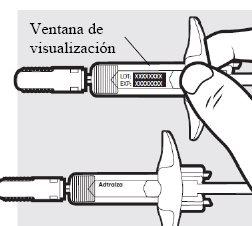
1e: Compruebe las2jeringas precargadas de Adtralza
- Asegúrese de que las etiquetas indican el nombre correcto del medicamento, Adtralza.
- Consulte la fecha de caducidad en las jeringas.
- Observe el medicamento a través de las ventanas de visualización. El medicamento debe ser de transparente a opalescente, de incoloro a amarillo pálido.
- No utilice las jeringas precargadas de Adtralza si:
- la fecha de caducidad en las jeringas ya ha pasado
- el medicamento se ve turbio, decolorado o contiene partículas
- las jeringas precargardas parecen dañadas o han sufrido alguna caída
Si no puede utilizar las jeringas, deséchelas en un contenedor para objetos punzantes y utilice jeringas nuevas.
- Es posible que observe pequeñas burbujas de aire en el líquido. Es normal; no tiene que hacer nada al respecto.
Paso2: Elección y preparación de la zona de inyección
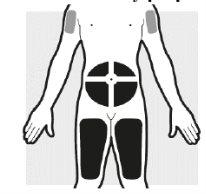

2a: Elija la zona en la que se administrarán las inyecciones
- Puede hacerlo en:
- la zona del vientre (abdomen)
- el muslo
- la parte superior del brazo, solo cuando el cuidador le administre las inyecciones.
- Noinyecte el medicamento en piel sensible, con hematomas, escamosa, con cicatrices, endurecida o con eccema.
- Noadministre la inyección a menos de 5 cm alrededor del ombligo.
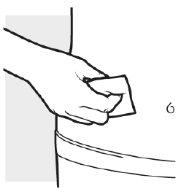
2b: Lávese las manos y prepare la piel
- Lávese las manos con agua y jabón.
- Limpie la zona elegida para las 2 inyecciones con una toallita con alcohol, realizando un movimiento circular.
- Deje que la zona se seque por completo.
- Nosople encima ni toque la zona limpia antes de la inyección.
Paso3: Inyección de Adtralza
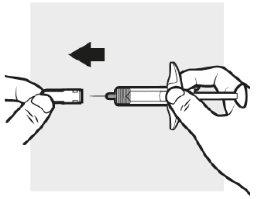
3a: Quite el capuchón de la aguja de Adtralza
Sujete el cuerpo de la jeringa precargada de Adtralza con una mano, y con la otra, tire hacia fuera del capuchón de la aguja y tírelo en un contenedor para objetos punzantes.
- Notrate de volver a tapar las jeringas precargadas de Adtralza.
- Nosostenga la varilla del émbolo o el cabezal del émbolo mientras retira el capuchón de la aguja.
- Puede que vea una gota de líquido en el extremo de la aguja. Es normal.
- Notoque la aguja ni deje que entre en contacto con ninguna superficie.
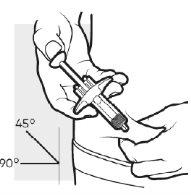
3b: Inserte la aguja
Con una mano pellizque suavemente y sostenga un pliegue de piel previamente limpia. Con la otra mano, introduzca la aguja completamente en la piel con un ángulo de entre 45 y 90 grados.
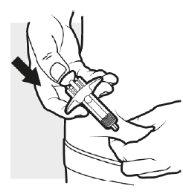
3c: Inyecte el medicamento
Empuje firmemente el cabezal del émbolo con el pulgar. Se habrá inyectado todo el medicamento cuando ya no pueda empujar más el émbolo.
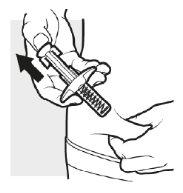
3d: Suelte y retire la aguja
Levante el pulgar del cabezal del émbolo. La aguja retrocederá automáticamente dentro del cuerpo de la jeringa y quedará dentro del cuerpo de la jeringa.
- Coloque una bolita de algodón o una gasa seca sobre la zona de inyección durante unos pocos segundos. No frote la zona de inyección. Si fuera necesario, cubra la zona de inyección con un pequeño apósito.
- Puede que haya una pequeña cantidad de sangre o líquido en el lugar de inyección. Es normal.
Deseche la jeringa precargada de Adtralza utilizada en un contenedor para objetos punzantes. Ver el paso5“Eliminación de Adtralza”.
Paso4: Inyección de la segunda jeringa

Para recibir la dosis prescrita completa, deberá administrarse una segunda inyección. Tome la segunda jeringa precargada de Adtralza y repita los pasos3y5.
Nota
Asegúrese de administrar la segunda inyecciónen la misma zona del cuerpo, pero a una distancia mínima de 3 cm con respecto a la primera.
Paso5: Eliminación de Adtralza
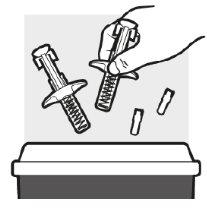
- Deseche las jeringas precargadas de Adtralza utilizadas en un contenedor para objetos punzantes justo después de su uso.
- Notire las jeringas precargadas de Adtralza junto a los residuos domésticos.
- Si no tiene ningún contenedor para objetos punzantes, puede hacer uno casero que:
- esté hecho de plástico resistente;
- pueda cerrarse con una tapa hermética y resistente a objetos punzantes, para que no asomen elementos afilados,
- se encuentre en posición recta y estable durante su uso,
- sea antifugas y
- esté correctamente etiquetado para advertir de los desperdicios peligrosos que contiene el envase.
- Cuando el envase resistente a objetos punzantes esté casi lleno, deberá seguir las directrices de su localidad para la eliminación inmediata de los contenedores para objetos punzantes.
- Norecicle el envase resistente a los pinchazos.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ADTRALZA 150 mg SOLUCION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, 300 mgPrincipio activo: TralokinumabFabricante: Leo Pharma A/SRequiere recetaForma farmacéutica: CAPSULA, 10 mgPrincipio activo: AlitretinoinaFabricante: Industrial Farmaceutica Cantabria S.A.Requiere recetaForma farmacéutica: CAPSULA, 30 mgPrincipio activo: AlitretinoinaFabricante: Industrial Farmaceutica Cantabria S.A.Requiere receta
Médicos online para ADTRALZA 150 mg SOLUCION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ADTRALZA 150 mg SOLUCION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes








