УПТРАВІ 800 МКГ ТАБЛЕТКИ, ПОКРИТІ ПЛІВКОВОЮ ОБОЛОНКОЮ

Запитайте лікаря про рецепт на УПТРАВІ 800 МКГ ТАБЛЕТКИ, ПОКРИТІ ПЛІВКОВОЮ ОБОЛОНКОЮ

Інструкція із застосування УПТРАВІ 800 МКГ ТАБЛЕТКИ, ПОКРИТІ ПЛІВКОВОЮ ОБОЛОНКОЮ
Введення
Опис: Інформація для пацієнта
Uptravi 200мікрограмів покритих таблеток
Uptravi 400мікрограмів покритих таблеток
Uptravi 600мікрограмів покритих таблеток
Uptravi 800мікрограмів покритих таблеток
Uptravi 1.000мікрограмів покритих таблеток
Uptravi 1.200мікрограмів покритих таблеток
Uptravi 1.400мікрограмів покритих таблеток
Uptravi 1.600мікрограмів покритих таблеток
селексіпаг
Цей лікарський засіб підлягає додатковому моніторингу, що полегшить виявлення нової інформації про його безпеку. Ви можете допомогти, повідомивши про будь-які побічні ефекти, які ви можете мати. Остання частина розділу 4 містить інформацію про те, як повідомляти про ці побічні ефекти.
Прочитайте уважно весь листок перед тим, як приймати цей лікарський засіб, оскільки він містить важливу інформацію для вас.
- Збережіть цей листок, оскільки вам може знадобитися знову його прочитати.
- Якщо у вас є якісь питання, проконсультуйтеся з вашим лікарем або медсестрою.
- Цей лікарський засіб призначений лише вам, і не давайте його іншим людям, навіть якщо вони мають такі самі симптоми, як у вас, оскільки це може їм нашкодити.
- Якщо ви відчуваєте побічні ефекти, проконсультуйтеся з вашим лікарем або медсестрою, навіть якщо це побічні ефекти, які не вказані в цьому листку (див. розділ 4).
Зміст листка
- Що таке Uptravi і для чого він використовується
- Що потрібно знати перед тим, як почати приймати Uptravi
- Як приймати Uptravi
- Можливі побічні ефекти
- Збереження Uptravi
- Зміст упаковки та додаткова інформація
1. Що таке Uptravi і для чого він використовується
Uptravi - це лікарський засіб, який містить активну речовину селексіпаг. Він діє на кровоносні судини подібно до природної речовини простацикліну, роблячи їх розслабленими та розширеними.
Uptravi використовується для тривалого лікування легеневої гіпертензії (ЛГ) у дорослих пацієнтів, які недостатньо контролюються іншими лікарськими засобами для ЛГ, відомими як антагоністи рецептора ендотеліну та інгібітори фосфодіестерази типу 5. Uptravi можна використовувати самостійно, якщо пацієнт не є кандидатом на ці лікарські засоби.
Легенева гіпертензія - це захворювання, характеризоване високим артеріальним тиском, який впливає на кровоносні судини, які транспортують кров від серця до легень (легеневі артерії). У людей з ЛГ ці судини є вужчими, тому серцю потрібно працювати більше, щоб перекачувати кров. Це може зробити так, що людина відчуває втому, головокружіння, труднощі з диханням або інші симптоми.
Так само, як і простациклін, Uptravi розширює легеневі артерії та зменшує їхнє звуження. Це робить серцю легше перекачувати кров через легеневі артерії. Знімає симптоми ЛГ та покращує перебіг захворювання.
2. Що потрібно знати перед тим, як почати приймати Uptravi
Не приймайте Uptravi
- якщо ви алергічні на селексіпаг або на інші компоненти цього лікарського засобу (перелічені в розділі 6).
- якщо у вас є порушення серця, такі як:
- зменшений кровоток до серцевих м'язів (сердечна недостатність); симптоми можуть включати біль у грудній клітці
- інфаркт міокарда за останні 6 місяців
- сердечна недостатність (декомпенсована серцева недостатність) без суворого медичного контролю
- важкі порушення серцевого ритму
- вади серцевих клапанів (вроджені або набуті), які роблять серце працювати з труднощами (не пов'язані з легеневою гіпертензією)
- якщо ви мали інсульт (інсульт) за останні 3 місяці або будь-які інші події, пов'язані з зменшенням кровотоку до мозку (наприклад, транзиторна ішемічна атака)
- якщо ви приймаєте гемфіброзил (лікарський засіб, який використовується для зниження рівня жирів у крові)
Попередження та застереження
Проконсультуйтеся з вашим лікарем або медсестрою перед тим, як почати приймати Uptravi, якщо:
- ви приймаєте лікарські засоби для лікування гіпертонії (високого артеріального тиску)
- у вас є низький артеріальний тиск, пов'язаний з симптомами, такими як головокружіння
- ви мали недавно значну кровотечу або втрату рідини, наприклад, важку діарею або блювоту
- у вас є проблеми з щитоподібною залозою
- у вас є важкі проблеми з нирками або ви проходите діаліз
- у вас є важкі проблеми з печінкою
Якщо ви відчуваєте будь-які з цих симптомів або ваше захворювання змінюється, повідомте своєму лікареві негайно.
Діти та підлітки
Не давайте цей лікарський засіб дітям молодшим 18 років, оскільки Uptravi не був оцінений у дітей.
Пацієнти похилого віку
Є обмежений досвід використання Uptravi у пацієнтів старших 75 років. Uptravi повинен бути використаний з обережністю у пацієнтів цієї вікової групи.
Інші лікарські засоби та Uptravi
повідомте своєму лікареві, якщо ви приймаєте, нещодавно приймали або плануєте приймати інші лікарські засоби.
Прийом інших лікарських засобів може вплинути на дію Uptravi.
повідомте своєму лікареві або медсестрі, якщо ви приймаєте будь-які з наступних лікарських засобів:
- Гемфіброзил (лікарський засіб, який використовується для зниження рівня жирів у крові)
- Клопідогрел (лікарський засіб, який використовується для профілактики утворення тромбів у коронарних артеріях)
- Деферасіrox (лікарський засіб, який використовується для видалення надлишку заліза з організму)
- Терифлуномід (лікарський засіб, який використовується для лікування рецидивної множинної склерози)
- Карбамазепін (лікарський засіб, який використовується для лікування певних типів епілепсії, невралгії або для допомоги в контролі важких порушень поведінки, коли інші лікарські засоби не діють)
- Фенітойн (лікарський засіб, який використовується для лікування епілепсії)
- Валпроєва кислота (лікарський засіб, який використовується для лікування епілепсії)
- Пробенецид (лікарський засіб, який використовується для лікування подагри)
- Флуконазол, рифампіцин або рифапентин (антібіотики, які використовуються для лікування інфекцій)
Вагітність та лактація
Не рекомендується використання Uptravi під час вагітності та лактації. Якщо ви жінка та можете завагітніти, ви повинні використовувати надійний метод контрацепції, поки приймаєте Uptravi. Якщо ви вагітні або перебуваєте у період лактації, вважаєте, що можете бути вагітною або плануєте завагітніти, проконсультуйтеся з вашим лікарем або фармацевтом перед тим, як прийняти цей лікарський засіб.
Водіння та використання машин
Uptravi може викликати побічні ефекти, такі як головний біль та зниження артеріального тиску (див. розділ 4), які можуть вплинути на вашу здатність водити транспортні засоби; симптоми вашого захворювання також можуть зменшити вашу здатність водити.
3. Як приймати Uptravi
Лікування Uptravi повинно бути розпочато та контролюватися лікарем, який має досвід у лікуванні легеневої гіпертензії (ЛГ). Слідуйте точно інструкціям щодо прийому цього лікарського засобу, вказаним вашим лікарем. Якщо у вас є питання, проконсультуйтеся з вашим лікарем знову.
повідомте своєму лікареві, якщо ви відчуваєте побічні ефекти, оскільки він може рекомендувати вам змінити дозу Uptravi.
повідомте своєму лікареві, якщо ви приймаєте інші лікарські засоби, оскільки він може рекомендувати вам приймати Uptravi тільки один раз на добу.
Якщо у вас поганий зір або ви відчуваєте будь-які види сліпоти, попросіть допомоги в іншої людини, щоб прийняти Uptravi під час періоду调整 дози.
Відповідна доза для вас
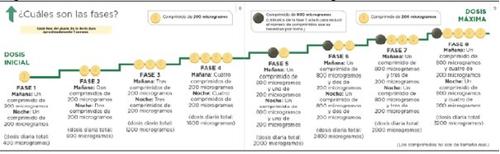
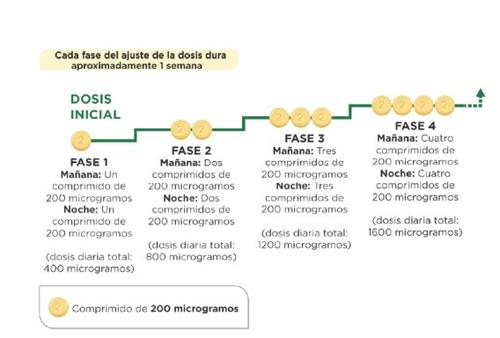
На початку лікування ви прийматимете найнижчу дозу. Це одна таблетка по 200мікрограміввранці та одна таблетка по 200мікрограмів увечері. Лікування повинно бути розпочато ввечері. Ваш лікар вказує вам, щоб ви поступово збільшували дозу. Це називається调整 дози, і дозволяє вашому організму адаптуватися до нового лікарського засобу. Метою调整 дози є досягнення найбільш відповідної дози. Це буде найвища доза, яку ви можете переносити, і може досягати максимальної дози 1.600 мікрограмів вранці та 1.600 мікрограмів увечері.
Перша упаковка таблеток, яку ви отримаєте, міститиме таблетки світло-жовтого кольору по 200 мікрограмів.
Ваш лікар вказує вам, щоб ви збільшували дозу поетапно, зазвичай кожну тиждень, хоча інтервал між збільшеннями може бути більшим.
На кожному етапі ви додаєте одну таблетку по 200 мікрограмів до ранкової дози та одну таблетку по 200 мікрограмів до вечірньої дози. Перший прийом збільшеної дози повинен бути зроблений увечері. Наступна схема показує кількість таблеток, яку ви повинні приймати кожного ранку та кожної вечеріу перших 4 етапах.
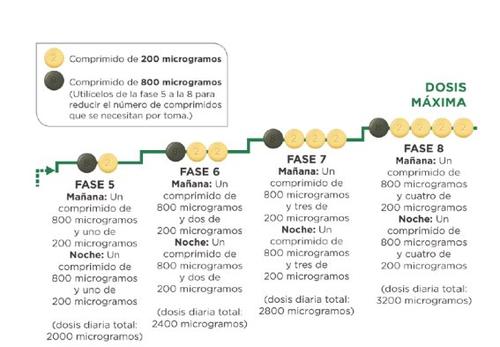
Якщо ваш лікар вказує вам продовжити збільшення дози та перейти до етапу 5, ви можете зробити це, прийнявши одну таблетку зеленого кольору по 800 мікрограмів та одну таблетку світло-жовтого кольору по 200 мікрограмів вранці та одну таблетку по 800 мікрограмів та одну таблетку по 200 мікрограмів увечері.
Якщо ваш лікар вказує вам продовжити збільшення дози, ви додаєте одну таблетку по 200 мікрограмів до ранкової дози та одну таблетку по 200 мікрограмів до вечірньої дози на кожному новому етапі. Перший прийом збільшеної дози повинен бути зроблений увечері. Максимальна доза Uptravi становить 1.600 мікрограмів вранці та 1.600 мікрограмів увечері. Однак не всі пацієнти досягнуть цієї дози, кожен пацієнт потребує окремої дози.
Наступна схема показує кількість таблеток, яку ви повинні приймати кожного ранку та кожної вечеріна кожному етапі, починаючи з етапу 5.
Упаковка для регулювання дози також містить посібник, який надає інформацію про процес регулювання дози та дозволяє вам записувати кількість таблеток, які ви приймаєте щодня.
Запам'ятайте записувати кількість таблеток, які ви приймаєте щодня, у вашому щоденнику регулювання дози. Етапи регулювання дози зазвичай тривають приблизно 1 тиждень. Якщо ваш лікар вказує вам продовжити кожний етап регулювання дози понад 1 тиждень, у вас є додаткові сторінки у щоденнику, які дозволяють вам зробити це. Запам'ятайте зв'язатися з вашим лікарем або медсестрою періодично під час етапу регулювання дози.
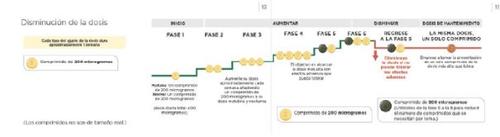
Зниження дози через побічні ефекти
Під час регулювання дози ви можете відчувати побічні ефекти, такі як головний біль, діарея, відчуття нудоти, блювота, біль у щелепі, біль у м'язах, біль у ногах, біль у суглобах або червоність обличчя, які ви не можете переносити або які не можна лікувати. Якщо ці побічні ефекти є для вас важкими, проконсультуйтеся з вашим лікарем щодо способів контролювати або лікувати їх. Є лікування, яке може допомогти вам полегшити ці побічні ефекти. Наприклад, анальгетики, такі як парацетамол, можуть допомогти вам лікувати біль та головний біль.
Якщо ці побічні ефекти не можна лікувати або вони не покращуються поступово з дозою, яку ви приймаєте, ваш лікар може регулювати дозу, зменшуючи кількість таблеток світло-жовтого кольору по 200 мікрограмів, які ви приймаєте, видаляючи одну таблетку вранці та одну таблетку увечері. Наступна схема показує, як зменшити дозу. Це повинно бути зроблено лише у випадку, якщо ваш лікар вказує вам зробити це.
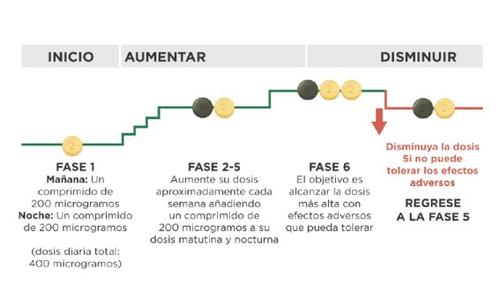
Якщо побічні ефекти, які ви відчуваєте, можна контролювати після зменшення дози, ваш лікар може вирішити, що вам потрібно продовжувати цю дозу. Для отримання додаткової інформації див. розділ "Доза підтримки" нижче.
Доза підтримки
Найвища доза, яку ви можете переносити під час етапу регулювання дози, стане вашою дозою підтримки. Ваш лікар призначить вам одну таблетку з відповідною потужністю для вашої дози підтримки. Це дозволяє вам приймати одну таблетку вранці та одну таблетку увечері, замість кількох таблеток кожен раз.
Для отримання повної інформації про таблетки Uptravi, включаючи їхній колір та гравіювання, див. розділ 6 цього листка.
З часом ваш лікар може регулювати вашу дозу підтримки, якщо це буде потрібно.
Якщо в будь-який момент, після прийому однієї й тієї ж дози протягом тривалого періоду, ви відчуваєте побічні ефекти, які ви не можете переносити або які впливають на вашу повсякденну діяльність, зв'яжіться з вашим лікарем, оскільки вам може знадобитися зменшення дози. Лікар може призначити вам одну таблетку з меншою концентрацією. Запам'ятайте викидати неприйняті таблетки (див. розділ 5).
Приймайте Uptravi один раз вранці та один раз увечері, з інтервалом приблизно 12 годин.
Приймайте таблетки з їжею, оскільки це може допомогти вам краще переносити лікарський засіб. Проглотіть таблетки цілими, допоможіть собі склянкою води.
Якщо ви прийняли більше Uptravi, ніж потрібно
Якщо ви прийняли більше таблеток, ніж потрібно, проконсультуйтеся з вашим лікарем негайно.
Якщо ви забули прийняти Uptravi
Якщо ви забули прийняти Uptravi, прийміть дозу якнайшвидше, коли ви про це пам'ятайте, та продовжуйте приймати таблетки за звичайним графіком. Якщо це майже час прийому наступної дози (за 6 годин до часу, коли ви зазвичай приймаєте її), ви повинні пропустити забуту дозу та продовжувати приймати лікарський засіб за звичайним графіком. Не приймайте подвійну дозу, щоб компенсувати забуту дозу.
Якщо ви припиняєте лікування Uptravi
Раптове припинення лікування Uptravi може зробити так, що ваші симптоми погіршаться. Не припиняйте приймати Uptravi, якщо ваш лікар не вказує вам зробити це. Ваш лікар може вказати вам зменшити дозу поступово перед тим, як припинити лікування повністю.
Якщо ви припиняєте приймати Uptravi протягом більше 3 днів поспіль (якщо ви забули 3 ранкові дози та 3 вечірні дози, або 6 доз поспіль чи більше), зв'яжіться з вашим лікарем негайно, оскільки вам може знадобитися регулювання дози, щоб уникнути побічних ефектів. Ваш лікар може вирішити знову розпочати лікування з меншої дози, щоб поступово збільшувати її до вашої дози підтримки.
Якщо у вас є будь-які інші питання щодо використання цього лікарського засобу, проконсультуйтеся з вашим лікарем або медсестрою.
4. Можливі побічні ефекти
Як і всі лікарські засоби, Uptravi може викликати побічні ефекти. Ви можете відчувати побічні ефекти не лише під час етапу регулювання дози, під час якого збільшується ваша доза, але також пізніше, після прийому однієї й тієї ж дози протягом тривалого періоду.
Якщо ви відчуваєте будь-які з наступних побічних ефектів: головний біль, діарея, відчуття нудоти, блювота, біль у щелепі, біль у м'язах, біль у ногах, біль у суглобах або червоність обличчя, які ви не можете переносити або які не можна лікувати, ви повинні зв'язатися з вашим лікарем, оскільки доза, яку ви приймаєте, може бути надто високою для вас і вам може знадобитися зменшення дози.
Дуже часті побічні ефекти(можуть виникнути у більше 1 з 10 осіб)
- Головний біль
- Червоність обличчя
- Нудота та блювота
- Діарея
- Біль у щелепі, біль у м'язах, біль у суглобах, біль у ногах
- Назофарингіт (конгестія носа)
Часті побічні ефекти(можуть виникнути у до 1 з 10 осіб)
- Анемія (низький рівень червоних кров'яних тілець)
- Гіпертиреоз (гіперактивна щитоподібна залоза)
- Зниження апетиту
- Втрата ваги
- Гіпотонія (низький артеріальний тиск)
- Біль у животі
- Біль
- Зміни в деяких аналізах, включаючи ті, які вимірюють рівень кров'яних клітин та функцію щитоподібної залози
- Висипання, включаючи кропив'янку, які можуть викликати відчуття печіння або свербіння та червоність шкіри
Побічні ефекти, які виникають рідше (можуть виникнути у до 1 з 100 осіб)
Збільшення частоти серцевих скорочень
Повідомлення про побічні ефекти
Якщо ви відчуваєте будь-які побічні ефекти, проконсультуйтеся з вашим лікарем, навіть якщо це побічні ефекти, які не вказані в цьому листку. Ви також можете повідомити про них безпосередньо через національну систему повідомлень, включену до Додатку V. Повідомляючи про побічні ефекти, ви можете допомогти надати більше інформації про безпеку цього лікарського засобу.
5. Збереження Uptravi
Тримайте цей лікарський засіб поза зоною досяжності дітей.
Не використовуйте Uptravi після закінчення терміну придатності, вказаного на упаковці та блистерній упаковці після "CAD". Термін придатності - це останній день місяця, який вказано.
Цьому лікарському засобу не потрібні особливі умови зберігання.
Не потрібно особливих заходів для видалення.
6. Зміст упаковки та додаткова інформація
Склад Уптраві
- Активний інгредієнт - селексіпаг.
Уптраві 200 мікрограмів таблетки, покриті оболонкою, містять 200 мікрограмів селексіпагу
Уптраві 400 мікрограмів таблетки, покриті оболонкою, містять 400 мікрограмів селексіпагу
Уптраві 600 мікрограмів таблетки, покриті оболонкою, містять 600 мікрограмів селексіпагу
Уптраві 800 мікрограмів таблетки, покриті оболонкою, містять 800 мікрограмів селексіпагу
Уптраві 1 000 мікрограмів таблетки, покриті оболонкою, містять 1 000 мікрограмів селексіпагу
Уптраві 1 200 мікрограмів таблетки, покриті оболонкою, містять 1 200 мікрограмів селексіпагу
Уптраві 1 400 мікрограмів таблетки, покриті оболонкою, містять 1 400 мікрограмів селексіпагу
Уптраві 1 600 мікрограмів таблетки, покриті оболонкою, містять 1 600 мікрограмів селексіпагу
- Інші компоненти:
У ядрі таблеток:
Манітол (Е421), кукурудзяний крохмаль, гідроксипропілцелюлоза низького заміщення, гідроксипропілцелюлоза та стеарат магнію.
У плівковому покритті:
Гіпромелоза, пропіленгліколь, діоксид титану (Е171), карнаубський віск та оксиди заліза (див. нижче).
Уптраві 200 мікрограмів таблетки, покриті оболонкою, містять жовтий оксид заліза (Е172).
Уптраві 400 мікрограмів таблетки, покриті оболонкою, містять червоний оксид заліза (Е172).
Уптраві 600 мікрограмів таблетки, покриті оболонкою, містять червоний та чорний оксид заліза (Е172).
Уптраві 800 мікрограмів таблетки, покриті оболонкою, містять жовтий та чорний оксид заліза (Е172).
Уптраві 1 000 мікрограмів таблетки, покриті оболонкою, містять червоний та жовтий оксид заліза (Е172).
Уптраві 1 200 мікрограмів таблетки, покриті оболонкою, містять чорний та червоний оксид заліза (Е172).
Уптраві 1 400 мікрограмів таблетки, покриті оболонкою, містять жовтий оксид заліза (Е172).
Уптраві 1 600 мікрограмів таблетки, покриті оболонкою, містять чорний, червоний та жовтий оксид заліза (Е172).
Вигляд Уптраві та вміст упаковки
Уптраві 200 мікрограмів таблетки, покриті оболонкою: таблетки, покриті оболонкою, світло-жовтого кольору, круглі, з позначенням «2» на одній стороні.
Уптраві 400 мікрограмів таблетки, покриті оболонкою: таблетки, покриті оболонкою, червоного кольору, круглі, з позначенням «4» на одній стороні.
Уптраві 600 мікрограмів таблетки, покриті оболонкою: таблетки, покриті оболонкою, світло-фіолетового кольору, круглі, з позначенням «6» на одній стороні.
Уптраві 800 мікрограмів таблетки, покриті оболонкою: таблетки, покриті оболонкою, зеленого кольору, круглі, з позначенням «8» на одній стороні.
Уптраві 1 000 мікрограмів таблетки, покриті оболонкою: таблетки, покриті оболонкою, оранжевого кольору, круглі, з позначенням «10» на одній стороні.
Уптраві 1 200 мікрограмів таблетки, покриті оболонкою: таблетки, покриті оболонкою, темно-фіолетового кольору, круглі, з позначенням «12» на одній стороні.
Уптраві 1 400 мікрограмів таблетки, покриті оболонкою: таблетки, покриті оболонкою, темно-жовтого кольору, круглі, з позначенням «14» на одній стороні.
Уптраві 1 600 мікрограмів таблетки, покриті оболонкою: таблетки, покриті оболонкою, коричневого кольору, круглі, з позначенням «16» на одній стороні.
Уптраві 200 мікрограмів таблетки, покриті оболонкою, випускаються в блистерних упаковках по 10 або 60 таблеток та 60 або 140 таблеток (упаковки для регулювання дози).
Уптраві 400 мікрограмів, 600 мікрограмів, 800 мікрограмів, 1 000 мікрограмів, 1 200 мікрограмів, 1 400 мікрограмів та 1 600 мікрограмів таблетки, покриті оболонкою, випускаються в блистерних упаковках по 60 таблеток.
Можливо, не всі ці форми будуть випускатися.
Уповноважений на отримання дозволу на продаж
Janssen-Cilag International NV
Turnhoutseweg 30
B-2340 Beerse
Бельгія
Відповідальний за виробництво
Actelion Manufacturing GmbH
Emil-Barell-Strasse 7
79639 Grenzach-Wyhlen
Німеччина
Actelion Pharmaceuticals Belgium NV
Bedrijvenlaan 1
2800 Mechelen
Бельгія
Для отримання додаткової інформації про цей лікарський засіб зверніться до місцевого представника уповноваженого на отримання дозволу на продаж:
Бельгія/Бельгія/Бельгія Actelion, підрозділ Janssen-Cilag International NV Тел./Телефон: +32-(0)15 284 777 | Литва Actelion, підрозділ Janssen-Cilag International NV Телефон: +370 5 278 68 88 |
Україна Actelion, підрозділ Janssen-Cilag International NV Телефон: +359 2 489 94 00 | Люксембург/Люксембург Actelion, підрозділ Janssen-Cilag International NV Тел./Телефон: +32-(0)15 284 777 |
Чехія Actelion, підрозділ Janssen-Cilag International NV Телефон: +420 221 968 006 | Угорщина Actelion, підрозділ Janssen-Cilag International NV Телефон: +36-1-413-3270 |
Данія Actelion, підрозділ Janssen-Cilag International NV Телефон: +45 3694 45 95 | Мальта Actelion, підрозділ Janssen-Cilag International NV Телефон: +356 2397 6000 |
Німеччина Actelion, підрозділ Janssen-Cilag International NV Телефон: +49 761 45 64 0 | Нідерланди Actelion, підрозділ Janssen-Cilag International NV Телефон: +31 (0)348 435950 |
Естонія Actelion, підрозділ Janssen-Cilag International NV Телефон: +372 617 7410 | Норвегія Actelion, підрозділ Janssen-Cilag International NV Телефон: +47 22480370 |
Греція Actelion, підрозділ Janssen-Cilag International NV Телефон: +30 210 675 25 00 | Австрія Actelion, підрозділ Janssen-Cilag International NV Телефон: +43 1 505 4527 |
Іспанія Actelion, підрозділ Janssen-Cilag International NV Телефон: +34 93 366 43 99 | Польща Actelion, підрозділ Janssen-Cilag International NV Телефон: +48 (22) 262 31 00 |
Франція Actelion, підрозділ Janssen-Cilag International NV Телефон: +33 (0)1 55 00 26 66 | Португалія Actelion, підрозділ Janssen-Cilag International NV Телефон: +351 214 368 600 |
Хорватія Actelion, підрозділ Janssen-Cilag International NV Телефон: + 385 1 6610 700 | Румунія Actelion, підрозділ Janssen-Cilag International NV Телефон: + 40 21 207 1800 |
Ірландія Actelion, підрозділ Janssen-Cilag International NV Телефон: +353 1 800 709 122 | Словенія Actelion, підрозділ Janssen-Cilag International NV Телефон: +386 1 401 18 00 |
Ісландія Actelion, підрозділ Janssen-Cilag International NV Телефон: +46 8 544 982 50 | Словаччина Actelion, підрозділ Janssen-Cilag International NV Телефон: +420 221 968 006 |
Італія Actelion, підрозділ Janssen-Cilag International NV Телефон: +39 0542 64 87 40 | Фінляндія Actelion, підрозділ Janssen-Cilag International NV Телефон: +358 9 2510 7720 |
Кіпр Actelion, підрозділ Janssen-Cilag International NV Телефон: +30 210 675 25 00 | Швеція Actelion, підрозділ Janssen-Cilag International NV Телефон: +46 8 544 982 50 |
Латвія Actelion, підрозділ Janssen-Cilag International NV Телефон: +371 678 93561 | Велика Британія Janssen-Cilag Ltd. Телефон: +44 1 494 567 444 |
Дата останнього перегляду цього посібника: листопад 2018 року
Детальна інформація про цей лікарський засіб доступна на сайті Європейського агентства з лікарських засобів: http://www.ema.europa.eu.
ПОСІБНИК ДЛЯ НАЛАГОДЖЕННЯ ДОЗИ: УПАКОВКА ДЛЯ НАЛАГОДЖЕННЯ ДОЗИ
Сторінка 1
Уптраві таблетки, покриті оболонкою селексіпаг Посібник для налаштування дози Початок лікування Уптраві Будь ласка, прочитайте доданий посібник перед початком лікування. Повідомте свого лікаря, якщо ви відчуваєте побічні ефекти, оскільки він може порадити вам змінити дозу Уптраві. Повідомте свого лікаря, якщо ви приймаєте інші лікарські засоби, оскільки він може порадити вам приймати Уптраві тільки один раз на день. |
Сторінка 2
Сторінка 3
Перелік Як приймати Уптраві? ................................................. 4 Як збільшити дозу? ............................................... 6 Які це фази? ..................................................... 8 Коли потрібно зменшити дозу? ............................ 10 Зменшення дози ............................................... 12 | Зміна на дозу підтримання ......................... 14 Якщо ви забули прийняти Уптраві ................................ 16 Якщо ви припинили лікування Уптраві ................... 17 Щоденник для налаштування дози ................................ 18 |
Сторінка 4
Сторінка 5
Як приймати Уптраві? Уптраві - лікарський засіб, який потрібно приймати вранціта ввечерідля лікування легеневого артеріального гіпертонуса, також званого ЛАГ. Початкова доза Уптраві становить 200 мікрограмів вранці та ввечері. Перший прийом Уптраві повинен бути здійснений ввечері. Ви повинні приймати кожну дозу з склянкою води, бажано під час їжі. | Є 2 фази лікування Уптраві: Налаштування дози Під час перших тижнів ваш лікар потребуватиме вашої співпраці, щоб знайти найвідповіднішою дозу Уптраві для вас. Ваш лікар може збільшити дозу від початкової дози. Ваш лікар може зменшити дозу. Цей процес називається налаштуванням дози, яке дозволяє вашому організму поступово адаптуватися до лікарського засобу. Підтримання Як тільки ваш лікар знайде найвідповіднішою дозу для вас, це буде доза, яку ви будете приймати звичайно. Це називається дозою підтримання. |
Сторінка 6
Сторінка 7
Як збільшити дозу? Лікування починається з дози 200 мікрограмів вранці та ввечері, і після обговорення з вашим лікарем або медсестрою збільшується доза до наступної фази. Перший прийом збільшеної дози повинен бути здійснений ввечері. Кожна фаза налаштування дози зазвичай триває приблизно 1 тиждень. Можливо, вам потрібно буде кілька тижнів, щоб знайти найвідповіднішою дозу для вас. Мета - досягти найвідповіднішої дози для вашого лікування. Ця доза буде вашою дозою підтримання. | Кожен пацієнт з ЛАГ є різним. Не всі пацієнти будуть мати одну й ту ж дозу підтримання. Деякі пацієнти можуть приймати 200 мікрограмів вранці та ввечері як дозу підтримання, тоді як інші досягнуть максимальної дози 1 600 мікрограмів вранці та ввечері. Інші можуть досягнути дози підтримання в якійсь точці між цими двома. Головне - досягти найвідповіднішої дози для вашого власного лікування. |
Сторінка 8
Сторінка 9
|
Сторінка 10
Сторінка 11
↓ЯКоли потрібно зменшити дозу? Як і з усіма лікарськими засобами, ви можете відчувати побічні ефекти при збільшенні дози Уптраві. Повідомте свого лікаря або медсестру, якщо ви відчуваєте побічні ефекти.Є лікування, яке може допомогти вам полегшити їх. Найбільш часті побічні ефекти (можуть впливати на більше 1 з 10 осіб), які ви можете відчувати під час прийому Уптраві, є:
Прочитайте посібник для отримання повного переліку побічних ефектів та додаткової інформації. | Якщо ви не можете терпіти побічні ефекти, навіть після того, як ваш лікар або медсестра намагалися їх вилікувати, він може порадити вам зменшити дозу. Якщо ваш лікар або медсестра порадив вам зменшити дозу, прийміть одну таблетку по 200 мікрограмів менше вранці та одну менше ввечері. Ви повинні зменшити дозу тільки після консультації з вашим лікарем або медсестрою. Цей процес зменшення дози допоможе вам знайти найвідповіднішою дозу для вас, також звану дозою підтримання. |
Сторінка 12
Сторінка 13
|
Сторінка 14
Сторінка 15
Зміна на дозу підтримання Найбільша доза, яку ви можете терпіти під час фази налаштування дози, стане вашою дозою підтримання.Ваш лікар або медсестра призначить вам одну таблетку з відповідною потужністюдля вашої дози підтримання. Це дозволяє вам приймати одну таблетку вранці та одну ввечері, замість кількох таблеток для кожної дози. | Наприклад, якщо ваша найбільша терпима доза під час фази налаштування дози становила 1 200 мікрограмів один раз вранці та один раз ввечері:
З часом ваш лікар або медсестра може змінити вашу дозу підтримання, якщо це буде необхідно. |
Сторінка 16
Сторінка 17
Якщо ви забули прийняти Уптраві Якщо ви забули прийняти одну дозу, прийміть її як тільки ви згадаєте, а потім продовжуйте приймати таблетки в звичайному графіку. Якщо ви згадаєте за 6 годин до часу, коли повинні прийняти наступну дозу, ви повинні відмовитися від прийому забутої дози та продовжувати приймати лікарський засіб в звичайному графіку. Не прийміть подвійну дозу, щоб компенсувати забуту дозу. | Якщо ви припинили лікування Уптраві Не припиняйте приймати Уптраві, якщо тільки ваш лікар або медсестра не порадив вам цього. Якщо ви припинили приймати Уптраві протягом більше 3 днів поспіль (якщо ви забули 6 доз поспіль або більше), зверніться до свого лікаря або медсестри негайно, оскільки вам може потрібно буде змінити дозу, щоб уникнути побічних ефектів. Ваш лікар або медсестра може вирішити знову почати лікування з меншої дози, щоб поступово збільшити її до вашої попередньої дози підтримання. |
Сторінка 18
Сторінка 19
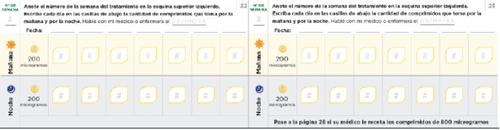
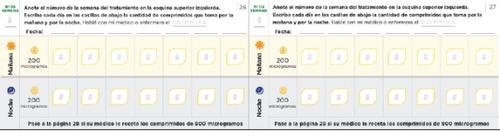
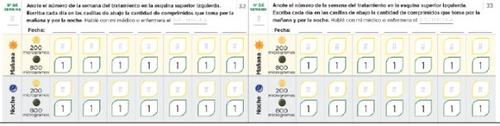
Щоденник для налаштування дози Читайте уважно інструкції, що містяться в посібнику. Наступні сторінки щоденника допоможуть вам вести облік кількості таблеток, які ви повинні приймати вранці та ввечері під час налаштування дози. Використовуйте їх, щоб записати кількість таблеток, які ви приймаєте вранці та ввечері. Кожна фаза зазвичай триває приблизно 1 тиждень, якщо тільки ваш лікар або медсестра не порадив вам інакше. Якщо фази налаштування дози тривають більше тижня, у вас є додаткові сторінки в щоденнику для реєстрації. Використовуйте сторінки 20-27 для реєстрації перших тижнів лікування, коли ви приймаєте тільки таблетки по 200 мікрограмів (фази 1-4). Якщо вам призначені таблетки як по 200, так і по 800 мікрограмів, використовуйте сторінки 30-37 (фази 5-8) | Пам'ятайте спілкуватися з вашим лікарем або медсестрою спеціалістом з ЛАГ регулярно. Запишіть вказівки вашого лікаря або медсестри: Телефон і електронна пошта лікаря: Телефон фармацевта: Нотатки: |
Сторінка 20
Сторінка 21
|
Сторінка 22
Сторінка 23
|
Сторінка 24Сторінка 25
|
Сторінка 26Сторінка 27
|
Сторінка 28Сторінка 29
Використовуйте наступні сторінки щоденника, якщо ваш лікар або медсестра призначила вам таблетки по 800 мікрограм разом з таблетками по 200 мікрограм. У сторінках щоденника перевірте, що ви прийняли однутаблетку по 800 мікрограм кожного дня ранку та ночі разом з кількістю таблеток по 200 мікрограм, призначених вам.
| Пам'ятайте спілкуватися зі своїм лікарем або медсестрою-спеціалістом з ВІЛ регулярно. Запишіть вказівки вашого лікаря або медсестри: Телефон і електронна пошта лікаря: Телефон фармацевта: Нотатки: |
Сторінка 30Сторінка 31
|
Сторінка 32Сторінка 33
|
Сторінка 34Сторінка 35
|
Сторінка 36Сторінка 37
|
Сторінка 38Сторінка 39
Нотатки |
Сторінка 40
Actelion Pharmaceuticals Ltd. |
- Країна реєстрації
- Діючі речовини
- Потрібен рецептТак
- Виробник
- Інформація є довідковою і не є медичною порадою. Перед прийомом будь-яких препаратів обов'язково проконсультуйтеся з лікарем. Oladoctor не несе відповідальності за медичні рішення, прийняті на основі цього контенту.
- Альтернативи до УПТРАВІ 800 МКГ ТАБЛЕТКИ, ПОКРИТІ ПЛІВКОВОЮ ОБОЛОНКОЮФорма випуску: ТАБЛЕТКА, 1000 мікрограмівДіючі речовини: selexipagВиробник: Janssen-Cilag International N.VПотрібен рецептФорма випуску: ТАБЛЕТКА, 1200 мікрограмДіючі речовини: selexipagВиробник: Janssen-Cilag International N.VПотрібен рецептФорма випуску: ТАБЛЕТКА, 1400 мікрограмДіючі речовини: selexipagВиробник: Janssen-Cilag International N.VПотрібен рецепт
Лікарі онлайн щодо УПТРАВІ 800 МКГ ТАБЛЕТКИ, ПОКРИТІ ПЛІВКОВОЮ ОБОЛОНКОЮ
Консультація щодо дозування, побічних ефектів, взаємодій, протипоказань та поновлення рецепта на УПТРАВІ 800 МКГ ТАБЛЕТКИ, ПОКРИТІ ПЛІВКОВОЮ ОБОЛОНКОЮ – за рішенням лікаря та згідно з місцевими правилами.