TIMOFTOL 5 mg/ml EYE DROPS SOLUTION

How to use TIMOFTOL 5 mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
TIMOFTOL 5 mg/ml eye drops, solution
Timolol
Read this package leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the package leaflet
- What is TIMOFTOL and what is it used for
- What you need to know before you use TIMOFTOL
- How to use TIMOFTOL
- Possible side effects
- Storage of TIMOFTOL
- Contents of the pack and other information
1. What is TIMOFTOL and what is it used for
Timolol is an ophthalmic beta-blocker agent that belongs to a group of medicines called topical anti-glaucoma agents.
This medicine is indicated to reduce high pressure in the eye in:
- ocular hypertension
- chronic open-angle glaucoma (including aphakic patients)
- some patients with secondary glaucoma.
2. What you need to know before you use TIMOFTOL
Do not use TIMOFTOL
- If you are allergic (hypersensitive) to timolol, beta-blockers or any of the other ingredients of Timoftol (listed in section 6).
- If you have now or have had in the past certain serious respiratory problems such as asthma.
- If you have now or have had in the past chronic obstructive pulmonary disease (severe lung disease that can cause wheezing, difficulty breathing and/or persistent cough).
- If you have certain heart diseases (such as slow or irregular heartbeats), sinus bradycardia (heart rate less than 60 beats per minute), second or third degree atrioventricular block, overt heart failure or cardiogenic shock.
- If you have corneal dystrophy (degenerative alteration of the cornea).
- If you have severe allergic rhinitis and bronchial hyperreactivity.
Warnings and precautions
Consult your doctor before starting to use Timoftol.
Be careful with Timoftol.
Before using this medicine, tell your doctor if you have or have had:
- heart problems such as coronary artery disease (symptoms include chest pain or tightness, shortness of breath or choking), heart failure or low blood pressure
- heart rhythm disorders (such as slow or irregular heartbeats)
- circulation problems (such as Raynaud's disease or Raynaud's syndrome)
- respiratory or lung problems (such as asthma or chronic obstructive pulmonary disease)
- diabetes or other blood sugar problems, as timolol may mask the signs and symptoms of low blood sugar
- hyperthyroidism or thyroid disease, as timolol may mask its signs and symptoms
Tell your doctor before surgery that you are using Timoftol, as it may change the effects of some medicines used during anesthesia.
Also, tell your doctor about any allergy or medication.
Timolol may be absorbed through the eye into the bloodstream, and the same side effects may occur as with the administration of other oral beta-blocker medicines.
- If you are taking oral beta-blockers or monoamine oxidase inhibitor antidepressants, tell your doctor, as they may increase the effects of Timoftol.
- It is not recommended to use two topical beta-blocker medicines at the same time.
- If you have breast cancer, Prinzmetal's angina, untreated pheochromocytoma, metabolic acidosis, severe peripheral circulatory disorders (Raynaud's disease) or low blood pressure.
- If you use contact lenses, it is not recommended to use Timoftol, as it increases the risk of intolerance to them.
As with any glaucoma treatment, it is recommended that your doctor regularly check the eye pressure and the condition of the cornea.
Use in athletes
This medicine contains timolol, which may produce a positive result in doping tests.
Children
As a general rule, eye drops containing timolol in solution should be used with caution in young patients. In the case of newborns, infants, and young children, timolol should be used with extreme caution. The use of this medicine should be stopped immediately if coughing, wheezing, abnormal breathing, or abnormal breathing pauses (apnea) appear. Inform your doctor immediately. A portable apnea monitor may be useful.
Timolol has been studied in infants and children aged between 12 days and 5 years with increased eye pressure or diagnosed glaucoma. For more information, consult your doctor.
Using TIMOFTOL with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines, including those obtained without a prescription.
Certain medicines may interact with Timoftol, including other eye drops for glaucoma treatment, and in these cases, it may be necessary to change the dose or stop treatment with either of them. It is important that you inform your doctor if you are taking any of the following medicines:
- Oral beta-blockers or blood pressure-lowering medicines, as they may increase the effects of timolol on intraocular pressure.
- Clonidine, as rebound high blood pressure may occur when clonidine treatment is stopped.
- Heart rhythm medicines, such as disopyramide, quinidine (also used to treat some types of malaria), and amiodarone, as timolol may increase their effects.
- Diabetes medicines (insulin and oral antidiabetics), as timolol may mask certain signs of hypoglycemia (low blood sugar) such as rapid heartbeat.
- Anesthetics.
- Stomach ulcer medicines, such as cimetidine.
- Alcohol.
- Epinephrine, as it may cause pupil dilation (mydriasis) when used with timolol.
- Antidepressant medicines, such as fluoxetine or paroxetine.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Do not use Timoftol if you are pregnant, unless your doctor considers it necessary. Due to the possible appearance of side effects in the fetus, your doctor will assess the benefit/risk of administering Timoftol.
Do not use Timoftol if you are breastfeeding. Timolol may pass into breast milk. Due to the possible appearance of side effects, your doctor will decide whether to stop treatment with Timoftol or stop breastfeeding.
Driving and using machines
Timoftol may cause dizziness, fatigue, or blurred vision, which may affect your ability to drive or use machines.
TIMOFTOL contains benzalkonium chloride and phosphates
This medicine contains 0.11 mg of benzalkonium chloride per ml.
Benzalkonium chloride can be absorbed by soft contact lenses and may alter their color. Remove contact lenses before using this medicine and wait 15 minutes before putting them back.
Benzalkonium chloride may cause eye irritation, especially if you have dry eye or other corneal diseases (the transparent layer on the front of the eye). Consult your doctor if you feel any unusual sensation, itching, or pain in the eye after using this medicine.
This medicine contains 30.42 mg of disodium hydrogen phosphate dodecahydrate and 6.10 mg of sodium dihydrogen phosphate dihydrate per ml. If you have severe damage to the transparent layer on the front of the eye (cornea), treatment with phosphates may, in rare cases, cause cloudy patches on the cornea due to calcium.
3. How to use TIMOFTOL
Follow the instructions for administration of this medicine indicated by your doctor. If in doubt, consult your doctor or pharmacist again.
Remember to use the medicine.
Your doctor will determine the dose and duration of treatment with Timoftol. Do not stop treatment prematurely, as this would stop its beneficial effect.
Timoftol is an eye drop for ophthalmic administration.
The normal dose is one drop of Timoftol 2.5 mg/ml in the affected eye(s) twice a day. If the response is not satisfactory, your doctor may increase the dose to one drop of Timoftol 5 mg/ml in the affected eye(s) twice a day.
Your doctor will periodically assess the response to treatment with Timoftol and decide whether it is necessary to supplement it with other medicines available to reduce intraocular pressure.
If you are using other eye drops at the same time, you should wait at least 10 minutes between applications so that the active ingredients are not eliminated from the eye.
In the case of this eye drop replacing another glaucoma treatment or being used in conjunction with other medicines, your doctor will indicate the regimen to follow.
If you think the action of Timoftol is too strong or too weak, tell your doctor or pharmacist.
Use in children and adolescents
Before using timolol, a complete medical examination should be performed. Your doctor will carefully assess the benefits versus the risks before considering treatment with timolol. If the benefits outweigh the risks, it is recommended to use the lowest available concentration of active substance once a day.
If the pressure is not sufficiently controlled with this concentration, it may be necessary to administer it twice a day with a 12-hour interval between them. Patients, especially newborns, should be closely monitored during the first one to two hours after the first administration, carefully watching for the appearance of side effects until surgery is performed. In the case of use in children, a concentration of 1 mg/ml of active substance may be sufficient to control pressure inside the eye, if available.
Duration of treatment
In the pediatric population, it will be prescribed as a temporary treatment.
Method of administration
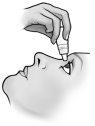
Only one drop of Timoftol should be instilled at each administration.
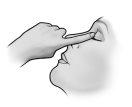
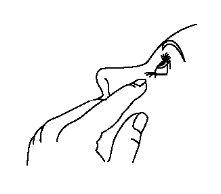
After instillation, keep your eyes closed for as long as possible (e.g., 3 to 5 minutes) and press the corner of your eye near your nose with your finger to prevent the drop of timolol from spreading to the rest of your body.
Instructions for use
Do not use the container if the plastic security strip around the neck of the container is not intact or is broken. When you open the container for the first time, tear off the plastic security strip.
[For containers other than OCUMETER PLUS:]
Every time you use Timoftol:
| |
| |
|
|
|
|
|
|
[Only for OCUMETER PLUS containers:]
- Before using the medicine for the first time, make sure the Security Strip on the front of the bottle is intact. When the bottle has not been opened yet, it is normal for there to be a space between the bottle and the cap.
Opening Arrows?
Safety Strip?
- Wash your hands. When you open the bottle for the first time, tear off the Safety Strip to break the seal.
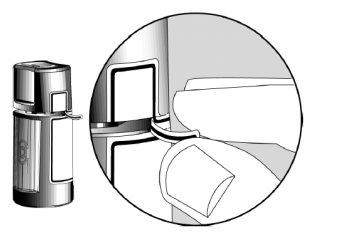
Space?
Finger Pressure Area?
- To open the bottle, unscrew the cap by turning it according to the arrows on the top of the cap. Do not pull the cap straight up. Pulling the cap up will prevent the dispenser from working properly.

Finger Pressure Area?
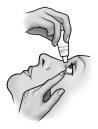
- Tilt your head back and slightly separate the lower eyelid, forming a small gap between the eyelid and the eye.
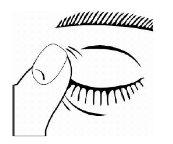
- Invert the bottle and gently press the "Finger Pressure Area" (as shown in the following figure) with your thumb or index finger until one drop is dispensed into the eye according to your doctor's instructions.
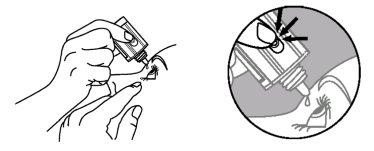
Finger Pressure Area
DO NOT TOUCH THE EYE OR EYELID WITH THE TIP OF THE DROPPER.
- After using Timoftol, press the corner of your eye near your nose with your finger (as shown in the following figure) for 2 minutes. This helps keep Timoftol in the eye and prevents timolol from spreading to the rest of your body.
- If, after opening for the first time, the drop dispensing is difficult, put the cap back on and squeeze (DO NOT SQUEEZE TOO HARD) and then remove the cap by turning it in the opposite direction, as indicated by the arrows on the top of the cap.
- Repeat steps 4 and 5 in the other eye if your doctor has told you to do so.
- Close the cap by turning it until it touches the edge of the bottle. For proper closure, the arrow on the left side of the cap should be aligned with the arrow on the left side of the bottle label. Do not squeeze too hard or you may damage the bottle and cap.
- The tip of the dispenser is designed to provide a single drop; therefore, DO NOT ENLARGE THE HOLE IN THE TIP OF THE DISPENSER.
- After you have used all the doses, some Timoftol will remain in the bottle. Do not worry, as an extra amount of Timoftol has been added, and you will get the total amount of Timoftol that your doctor has prescribed. Do not try to extract the excess medicine from the bottle.
Ophthalmic medicines, if used improperly, can become contaminated with common bacteria known to cause eye infections. The use of contaminated ophthalmic solutions can lead to serious eye disorders and subsequent loss of vision. If you think your medication may be contaminated, or if you develop an eye infection, contact your doctor immediately about continuing to use that bottle.
If you use more TIMOFTOL than you should
If you have used more Timoftol than you should, consult your doctor or pharmacist immediately.
The most common symptoms in case of overdose with timolol are: dizziness, headache, shortness of breath, decrease in heart rate, decrease in blood pressure, heart failure, and/or cardiac arrest.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91.562.04.20, indicating the medicine and the amount used.
If you forget to use TIMOFTOL
Do not use a double dose to make up for forgotten doses.
Use Timoftol according to the regimen indicated by your doctor. If you forget a dose, administer it as soon as possible. However, if it is almost time for the next dose, skip the forgotten dose and return to your usual administration schedule.
4. Possible Adverse Effects
Like all medicines, Timoftol can cause adverse effects, although not all people suffer from them.
Normally, you will be able to continue using the drops, unless the effects are severe. If you are concerned, consult your doctor or pharmacist. Do not stop using Timoftol without discussing it with your doctor first.
With the administration of timolol via the ocular route, the following adverse effects have been observed:
Frequent (may affect up to 1 in 10 patients):
- headache
- signs and symptoms of ocular irritation (e.g., burning sensation, stinging, itching, conjunctivitis, tearing, redness), eyelid inflammation, corneal inflammation, decreased corneal sensitivity, and dry eyes
Infrequent (may affect up to 1 in 100 patients):
- depression
- dizziness, fainting
- visual disturbances such as refractive changes
- slow heart rate (bradycardia)
- difficulty breathing (dyspnea)
- nausea, heavy digestion (dyspepsia)
- fatigue
Rare (may affect up to 1 in 1,000 patients):
- signs and symptoms of allergic reactions including angioedema (swelling under the skin in areas such as the face and limbs, which can obstruct the airways and cause difficulty swallowing or breathing), urticaria or hives with itching, localized and generalized rash, anaphylaxis (severe allergic reaction that can be life-threatening)
- insomnia (difficulty sleeping), nightmares, memory loss
- tingling or cramping sensation, increased signs and symptoms of myasthenia gravis (muscular disorder), decreased sexual appetite (decreased libido), stroke (cerebrovascular accident), cerebral ischemia (reduced blood flow to the brain)
- drooping eyelid (ptosis), double vision (diplopia), choroidal detachment (detachment of the vascular layer under the retina after filtration surgery, which can cause vision changes)
- tinnitus (ringing in the ears)
- chest pain, palpitations, swelling, irregular heart rhythm, congestive heart failure (disease characterized by difficulty breathing and swelling of feet and legs due to fluid accumulation), cardiac block, cardiac arrest
- low blood pressure, Raynaud's phenomenon (disorder of blood vessels that usually affects the fingers and toes), pain or discomfort in a limb when starting to walk, cold hands and feet
- breathing problems and constriction of airways (predominant in patients with pre-existing bronchospastic disease), cough
- diarrhea, dry mouth
- hair loss, psoriasiform skin rash or exacerbation of psoriasis
- inflammatory disease with fever, weakness, joint pain, and skin lesions (systemic lupus erythematosus)
- Peyronie's disease (which can cause a marked curvature of the penis)
Frequency not known (cannot be estimated from available data):
- hallucinations
Like other eye medications, timolol passes into the bloodstream. This can cause adverse effects similar to those observed with beta-blockers administered orally or by injection. The occurrence of adverse effects via topical ocular administration is less frequent than in the case of oral or injectable administration. The listed adverse effects include those that have been observed in the class of beta-blockers used to treat eye diseases:
- Generalized allergic reactions including swelling under the skin in areas such as the face and limbs, which can obstruct the airways and cause difficulty swallowing or breathing, urticaria or hives with itching, localized and generalized rash, severe allergic reaction that can be life-threatening.
- Low blood glucose level.
- Difficulty sleeping (insomnia), depression, nightmares, memory loss.
- Fainting, stroke (cerebrovascular accident), reduced blood flow to the brain, worsening of signs and symptoms of myasthenia gravis (muscular disorder), dizziness, strange sensations such as cramps and headache.
- Signs and symptoms of ocular irritation (e.g., burning, stinging, itching, tearing, redness), eyelid inflammation, corneal inflammation, blurred vision, and detachment of the vascular layer under the retina after filtration surgery, which can cause vision changes, decreased corneal sensitivity, dry eyes, corneal erosion (damage to the front layer of the eyeball), drooping eyelid (ptosis), double vision.
- Slow heart rate, palpitations, chest pain, edema (fluid accumulation), changes in heart rate or rhythm, congestive heart failure (disease characterized by difficulty breathing and swelling of feet and legs due to fluid accumulation), cardiac arrhythmia, cardiac block, cardiac arrest.
- Low blood pressure, Raynaud's phenomenon, cold hands and feet.
- Constriction of airways (predominant in patients with pre-existing disease), difficulty breathing, cough.
- Altered taste, nausea, indigestion, diarrhea, dry mouth, abdominal pain, vomiting.
- Hair loss, psoriasiform skin rash or exacerbation of psoriasis, skin rash.
- Muscle pain not caused by exercise.
- Sexual dysfunction, decreased libido.
- Muscle weakness/fatigue.
Reporting of Adverse Effects:
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that does not appear in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es.
By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Storage of TIMOFTOL
Keep this medication out of sight and reach of children.
Do not store at a temperature above 25°C.
Keep in the original packaging to protect it from light.
Do not use this medication after the expiration date shown on the packaging after the abbreviation CAD.
The expiration date is the last day of the month indicated.
Discard four weeks after opening the packaging.
Medicines should not be thrown away through wastewater or household waste. Deposit the packaging and unused medicines at the SIGRE point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and unused medicines. This way, you will help protect the environment.
6. Packaging Contents and Additional Information
Composition of TIMOFTOL
- The active ingredient is timolol. Each ml of Timoftol contains 6.8 mg of timolol maleate, equivalent to 5 mg of timolol.
- The other ingredients are sodium dihydrogen phosphate dihydrate, disodium hydrogen phosphate dodecahydrate, sodium hydroxide, benzalkonium chloride, and water for injectable preparations.
Appearance of the Product and Packaging Contents
Timoftol is presented as a clear, colorless or light yellow eye drop solution.
It is available in two alternative packaging options:
- Packaging with a dropper cap containing 5 ml of solution.
- Ocumeter Plus ocular dispenser containing 3 ml of solution.
Only some packaging sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Santen Oy
Niittyhaankatu 20
33720 Tampere
Finland
Manufacturer
Laboratoires Merck Sharp & Dohme
Chibret (“MIRABEL PLANT”)
Route de Marsat, RIOM
63963 Clermont – Ferrand, Cedex 9, France
Or
Santen Oy
Kelloportinkatu 1
33100 Tampere
Finland
Local Representative
Santen Pharmaceutical Spain S.L.
Acanto, 22, 7º
28045 – Madrid
Spain
Date of the Last Revision of this Leaflet: April 2020
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TIMOFTOL 5 mg/ml EYE DROPS SOLUTIONDosage form: EYE DROP, 5 mg/mlActive substance: timololManufacturer: Immedica Pharma AbPrescription requiredDosage form: OPHTHALMIC GEL, 1 mg/gActive substance: timololManufacturer: Sifi S.P.A.Prescription requiredDosage form: EYE DROP, 0.25%Active substance: timololManufacturer: Laboratorios Thea S.A.Prescription required
Online doctors for TIMOFTOL 5 mg/ml EYE DROPS SOLUTION
Discuss questions about TIMOFTOL 5 mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions