
SMOFLIPID 200 mg/ml EMULSION FOR INFUSION


How to use SMOFLIPID 200 mg/ml EMULSION FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
SMOFlipid 200 mg/ml emulsion for infusion
Soybean seed oil, medium-chain triglycerides, olive oil, fish oil
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again. If you have any further questions, ask your doctor, pharmacist, or nurse.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is SMOFlipid and what is it used for
- What you need to know before you take SMOFlipid
- How to take SMOFlipid
- Possible side effects
- Storage of SMOFlipid
- Contents of the pack and further information
1. What is SMOFlipid and what is it used for
SMOFlipid contains four different lipids (fats): soybean seed oil, medium-chain triglycerides, olive oil, and fish oil, which is rich in omega-3 fatty acids. The liquid is a mixture of fats and water, which is called a "lipid emulsion".
- it works by providing energy and fatty acids to the body
- it is introduced into your blood through a drip or infusion pump
A healthcare professional will give you SMOFlipid when other forms of feeding are not suitable or have not worked.
2. What you need to know before you take SMOFlipid
Do not use SMOFlipid
- if you are allergic to soybean seed oil, medium-chain triglycerides, olive oil, fish oil, or any of the other ingredients of this medicine (listed in section 6)
- if you are allergic (hypersensitive) to other products containing fish, egg, soy, or peanut.
- if you have too much fat in your blood (called "severe hyperlipemia")
- if you have severe kidney or liver problems
- if you have severe blood coagulation disorders (called "coagulation disorders")
- if you are in acute shock
- if you have fluid in your lungs (called "pulmonary edema"), fluid overload (hyperhydration), and heart failure (due to excess fluid in the body)
- if you are in an unstable condition, for example, after severe injuries, acute myocardial infarction, stroke, blood clotting (thrombosis), metabolic acidosis (metabolic disorder resulting in high acid levels in the blood), or untreated diabetes, blood poisoning, and dehydration.
Warnings and precautions
Talk to your doctor or nurse before starting SMOFlipid if you have a problem with high lipid levels in your blood due to your body not being able to use fats properly (called "altered lipid metabolism").
When used in newborns and children under 2 years, the solution (in the bags and administration equipment) should be protected from light exposure until the end of administration. Exposure of SMOFlipid to ambient light, especially after mixing with oligoelements or vitamins, generates peroxides and other degradation products that can be reduced if the product is protected from light exposure.
Allergic reactions
If you have any allergic reaction while receiving SMOFlipid, it is necessary to stop it immediately. If you experience any of the following situations while receiving the infusion, tell your doctor or nurse immediately:
- fever (high temperature)
- shivering
- hives
- difficulty breathing
Children
Tell your doctor or nurse if this medicine is being given to your newborn baby and they have:
- too much of a substance called "bilirubin" in the blood (hyperbilirubinemia)
- high blood pressure in their lungs (pulmonary hypertension)
If your newborn baby is taking SMOFlipid for a long time, the doctor will do blood tests to see how it is working.
Using SMOFlipid with other medicines:
Tell your doctor or pharmacist if you are using or have recently used other medicines.
In particular, tell your doctor if you are taking or have recently taken medicines used to stop blood clotting such as warfarin and heparin.
- SMOFlipid naturally contains vitamin K, which may affect warfarin. However, the content of vitamin K1 in SMOFlipid is so low that such problems are very rare.
- Heparin administered at clinical doses may cause an initial increase in blood fatty acid levels due to the release of fatty acids from tissues into the bloodstream and therefore fewer fatty acids will be eliminated from your blood (decreased triglyceride clearance).
Pregnancy and breastfeeding:
It is not known if it is safe to receive SMOFlipid while pregnant or breastfeeding. If you need to have intravenous feeding during pregnancy or breastfeeding, your doctor will only give you SMOFlipid after careful consideration.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
Driving and using machines:
This is not relevant because the medicine is administered in the hospital.
SMOFlipid contains sodium
This medicine contains 5 mmol (115 mg) of sodium per 1000 ml. Patients on a low-sodium diet should be aware of this.
3. How to take SMOFlipid
SMOFlipid is given into your blood through a drip or infusion pump. Your doctor will decide your dose based on your body weight and your ability to use the amount of fat being infused.
When used in newborns and children under 2 years, the solution (in the bags and administration equipment) should be protected from light exposure until the end of administration (see section 2).
For doctors and healthcare professionals, see "Method of administration" at the end of the package leaflet for more details on dosage and administration.
If you take more SMOFlipid than you should
If the dose you receive is too high, there is a risk that the amount of fat you receive is greater than your body can handle. This is called "fat overload syndrome". For more information, see section 4, Possible side effects.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately, or call the Toxicological Information Service, phone 91 562 04 20 (indicating the medicine and the amount ingested)
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Fat overload syndrome.
This can happen when your body has problems using fats due to too much SMOFlipid. It can also happen due to a sudden change in your condition (such as kidney problems or infection). Fat overload syndrome is characterized by high levels in the blood (hyperlipidemia), fever, more fat than normal in your tissues (fat infiltration), and disorders in various organs of the body and coma. All symptoms will normally disappear when treatment is stopped.
Common (may affect up to 1 in 10 patients)
- mild increase in body temperature
Uncommon (may affect up to 1 in 100 patients)
- shivering
- loss of appetite
- nausea
- vomiting
Rare (may affect up to 1 in 1,000 patients)
- allergic reactions (e.g., high temperature, inflammation, decreased blood pressure, skin rash, redness, headache)
- feeling of cold and heat
- pallor
- bluish discoloration of the skin and mucous membranes (due to a reduction in oxygen content in the blood)
- pain in the neck, back, bones, chest, and lower back
- high or low blood pressure
- difficulty breathing
Very rare (may affect up to 1 in 10,000 patients)
- prolonged and convulsive erection in men
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Surveillance System for Human Use: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of SMOFlipid
Keep out of sight and reach of children.
Do not store above 25°C. Do not freeze.
Do not use SMOFlipid after the expiry date stated on the label after EXP. The expiry date is the last day of the month indicated.
Do not use SMOFlipid if you notice that the packaging is damaged. Use only if the solution is white and homogeneous. For single use. The unused product should be discarded. Do not reuse.
Ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
When used in newborns and children under 2 years, the solution (in the bags and administration equipment) should be protected from light exposure until the end of administration (see section 2).
6. Contents of the pack and further information
Composition of SMOFlipid:
The active ingredients are:
Soybean oil, refined 60 mg/ml
Medium-chain triglycerides 60 mg/ml
Olive oil, refined 50 mg/ml
Fish oil, rich in omega-3 fatty acids 30 mg/ml
The other ingredients are: Glycerol, egg lecithin, dl-α-tocopherol (vitamin E), water for injections, sodium hydroxide (for pH adjustment), sodium oleate
Appearance of the product and pack contents
SMOFlipid is a white and homogeneous emulsion and is available in glass bottles or plastic bags
Glass bottle Plastic bag
100 ml 100 ml
10x100 ml 10x100 ml, 20 x100 ml
250 ml 250 ml
10x250 ml 10x250 ml
500 ml 500 ml
10x500 ml 10x500 ml
1000 ml
6x1000 ml
Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer:
Marketing authorisation holder
Fresenius Kabi AB
751 74 Uppsala
Sweden
Local representative
Fresenius Kabi España S.A.U
Marina 16-18 08005 (Barcelona)
Spain
Manufacturer
Fresenius Kabi AB, SE-751 74 Uppsala, Sweden (plastic bags)
Fresenius Kabi Austria GmbH, A-8055 Graz, Austria (glass bottles)
You can ask for more information about this medicine from the local representative of the marketing authorisation holder:
This medicine is authorised in the Member States of the European Economic Area under the following names:
Austria, Belgium, Finland, France, Germany, Iceland, Ireland, Italy, Netherlands, Norway, Slovenia, Sweden, United Kingdom: SMOFlipid 200 mg/ml
Cyprus, Czech Republic, Estonia, Greece, Hungary, Latvia, Lithuania, Luxembourg, Spain: SMOFlipid 20%
Denmark, Poland, Portugal, Slovakia: SMOFlipid
Date of last revision of this leaflet: February 2020
Detailed and up-to-date information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
-----------------------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
Warnings and precautions
The serum triglyceride concentration should not exceed 3 mmol/l during infusion. An overdose can cause fat overload syndrome. Special caution is required in patients with a marked risk of hyperlipidemia (e.g., patients with high lipid doses, severe sepsis, and babies with extremely low birth weight).
Administration of medium-chain fatty acids alone may result in metabolic acidosis. This risk is largely eliminated by the simultaneous infusion of long-chain fatty acids included in SMOFlipid. Additionally, simultaneous administration of carbohydrates will also eliminate this risk. Therefore, it is recommended to infuse a carbohydrate solution or carbohydrates with amino acids simultaneously. Routine laboratory tests associated with monitoring intravenous nutrition should be checked regularly. These include blood glucose levels, liver function tests, acid-base metabolism, fluid balance, complete blood count, and electrolytes.
Any sign or symptom of anaphylactic reaction (such as fever, shivering, hives, or shortness of breath) should be followed by immediate interruption of the infusion.
SMOFlipid should be administered with caution in neonates and premature infants with hyperbilirubinemia and in cases of pulmonary hypertension. In neonates, particularly in premature infants with long-term parenteral nutrition, platelet counts, liver function tests, and serum triglycerides should be monitored.
SMOFlipid contains up to 5 mmol of sodium per 1000 ml. This should be taken into account in patients on a low-sodium diet.
In general, it is recommended to avoid adding other medicines or substances to SMOFlipid unless their compatibility is known.
Method of administration
Intravenous infusion into a peripheral or central vein.
Instructions for use and handling
Use only if the emulsion is homogeneous. For the infusion bag: The integrity indicator (Oxalert) should be inspected before removing the overbag. If the indicator is black, oxygen has entered the overbag and the product should be discarded. Check the emulsion visually for any phase separation before administration. Ensure that the final emulsion for infusion does not show any signs of phase separation. For single use. Any remaining mixture should be discarded.
Additives:SMOFlipid can be mixed aseptically with amino acids, glucose, and electrolyte solutions to produce Total Parenteral Nutrition "All-In-one" (TPN) mixtures. On request, compatibility data with different additives and storage times for different mixtures are available. Additions should be made aseptically. Any remaining mixture after infusion should be discarded.
Do not store above 25°C. Do not freeze
Stability after mixing
In the event of mixing SMOFlipid with other products, the mixture should be used immediately from a microbiological point of view. If the mixtures are not used immediately, the time after opening the container and storage conditions are the responsibility of the user and should not normally exceed 24 hours at 2-8°C, unless the additions have been made under controlled and validated aseptic conditions.
Instructions for use for the infusion bag only
Instructions for use:SMOFlipid 200 mg/ml emulsion for infusion
Fresenius Kabi infusion bags
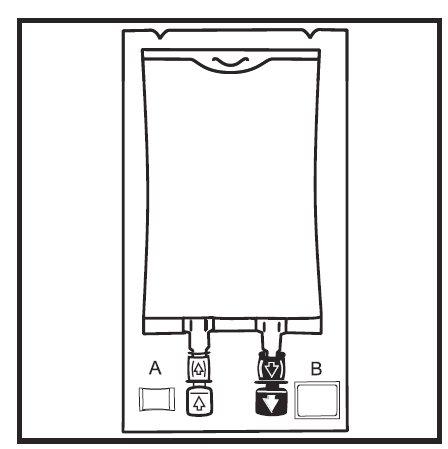
- Inspect the integrity indicator (Oxalert) A before removing the overbag. If the indicator is black, oxygen has entered the overbag and the product should be discarded.
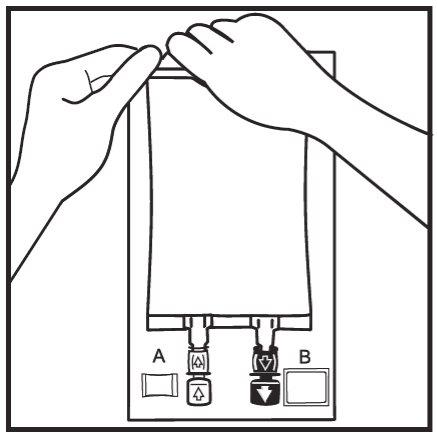
- Remove the overbag by tearing along the notch and pulling downwards along the container. The Oxalert bag A and the oxygen absorber B should be discarded.
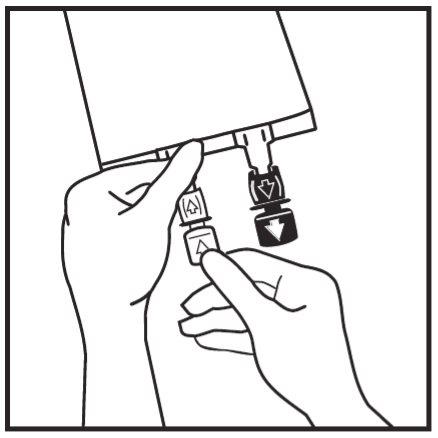
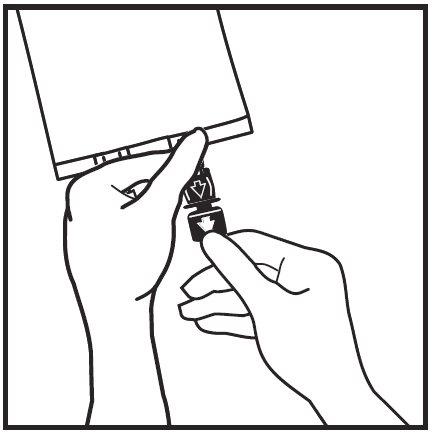
- If additives are required, break the white addition port by the arrow mark (A). If no additives are required, go to step 5.
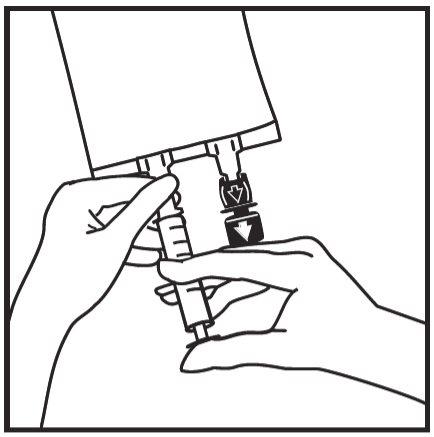
- Insert the needle horizontally through the center of the addition port membrane and inject the additives (of known compatibility). Use syringes with needles of 18-23 gauge and a maximum length of 40 mm.
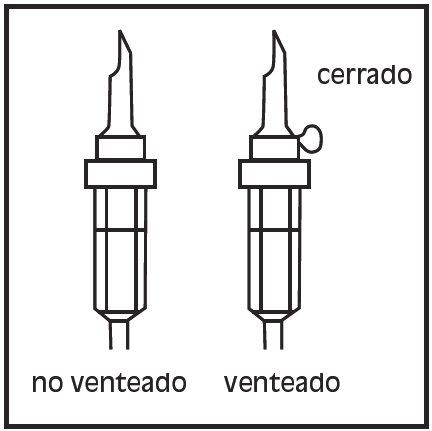
- Use an infusion set without air intake or close the air intake of the infusion set. Follow the instructions for use of the infusion set. Use a tip with the specified diameter in ISO 8536-4, 5.6 +/- 0.1 mm.
- Break the blue infusion port by the arrow mark.
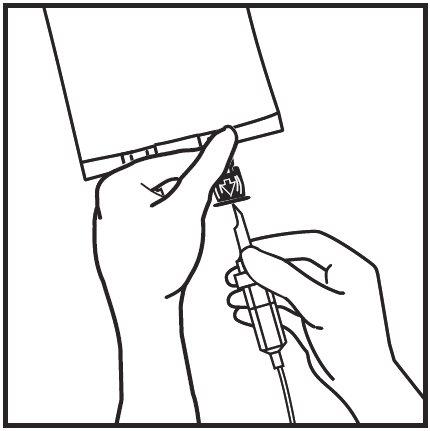
- Hold the base of the infusion port. Insert the tip through the infusion port with a slight wrist rotation until the tip is fully inserted.
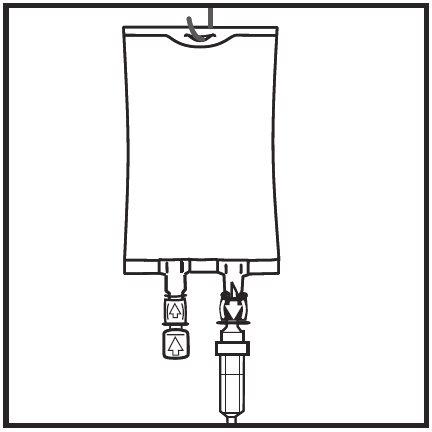
Hang the bag on the hanger by the perforated notch and start the infusion
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SMOFLIPID 200 mg/ml EMULSION FOR INFUSIONDosage form: INJECTABLE PERFUSION, 200 mg/mlActive substance: fat emulsionsManufacturer: Baxter S.L.Prescription requiredDosage form: INJECTABLE PERFUSION, 20 gActive substance: fat emulsionsManufacturer: Fresenius Kabi España, S.A.U.Prescription requiredDosage form: INJECTABLE PERFUSIONActive substance: fat emulsionsManufacturer: B Braun Medical S.A.Prescription required
Online doctors for SMOFLIPID 200 mg/ml EMULSION FOR INFUSION
Discuss questions about SMOFLIPID 200 mg/ml EMULSION FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















