
RYALTRIS 25 MICROGRAMOS/600 MICROGRAMOS/PULSACION SUSPENSION PARA PULVERIZACION NASAL

Cómo usar RYALTRIS 25 MICROGRAMOS/600 MICROGRAMOS/PULSACION SUSPENSION PARA PULVERIZACION NASAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Ryaltris 25 microgramos/ 600 microgramos/ pulsación, suspensión para pulverización nasal
mometasona furoato/olopatadina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Ryaltris y para qué se utiliza
- Qué necesita saber antes de empezar a usar Ryaltris
- Cómo usar Ryaltris
- Posibles efectos adversos
- Conservación de Ryaltris
- Contenido del envase e información adicional
1. Qué es Ryaltris y para qué se utiliza
Ryaltris contiene dos principios activos: furoato de mometasona y olopatadina.
- El furoato de mometasona pertenece a un grupo de medicamentos denominados corticosteroides (esteroides) que reducen la inflamación, que a menudo aparece en la rinitis alérgica.
- La olopatadina pertenece a un grupo de medicamentos denominados antihistamínicos. Los antihistamínicos actúan previniendo los efectos de sustancias como la histamina que el cuerpo produce como parte de una reacción alérgica, reduciendo así los síntomas de la rinitis alérgica.
Ryaltris se utiliza para tratar los síntomas de la rinitis alérgica estacional de moderada a grave(también llamada fiebre del heno) y la rinitis perenneen adultos y adolescentes de 12 años y mayores.
La rinitis alérgica estacional(fiebre del heno) es una reacción alérgica que se produce en ciertas épocas del año y es causada por la inhalación del polen de los árboles, hierbas, malezas y también mohos y esporas de hongos.
La rinitis perennese presenta durante todo el año y los síntomas pueden ser causados por una sensibilidad a una variedad de cosas incluyendo los ácaros del polvo, pelo del animal (o caspa), plumas y ciertos alimentos.
Ryaltris alivia los síntomas de las alergias, como la secreción nasal, los estornudos, el picor y la congestión nasal.
2. Qué necesita saber antes de empezar a usar Ryaltris
No use Ryaltris
- si es alérgicoal furoato de mometasona, la olopatadina o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si presenta una infección nasal no tratada. El uso de Ryaltris mientras tiene una infección de nariz no tratada, como el herpes, puede empeorar la infección. Debe esperar hasta que la infección desaparezca antes de comenzar a usar el pulverizador nasal.
- si ha sido sometido a una operacióno ha tenido una lesión reciente en la nariz. No debe utilizar el pulverizador nasal hasta que haya cicatrizado la nariz.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar Ryaltris
- si tiene o ha tenido alguna vez tuberculosis.
- si tiene cualquier otra infección.
- si está tomando otros corticosteroides, ya sea por vía oral o mediante inyección.
Consulte a su médico o farmacéutico mientras esté utilizando Ryaltris
- si tiene dificultades en superar las infecciones (su sistema inmunológico no está funcionando bien) y entra en contacto con alguien con sarampión o varicela. Debe evitar el contacto con cualquier persona que tenga estas infecciones.
- si tiene infección de nariz o garganta.
- si lleva usando este medicamento durante varios meseso más tiempo.
- si tiene irritación persistente de nariz o garganta.
- si tiene visión borrosau otras alteraciones visuales.
Si los pulverizadores nasales de corticosteroides se usan a dosis altas durante largos periodos de tiempo, se pueden producir efectos adversos, debido a que el medicamento se absorbe en el organismo. Estos efectos adversos pueden incluir pérdida de peso, fatiga, debilidad muscular, niveles bajos de azúcar en sangre, niveles bajos de sodio, dolor en las articulaciones, depresión y oscurecimiento de la piel. Si le aparece alguno de estos efectos adversos, su médico puede recomendarle otro medicamento durante períodos de estrés o cirugías programadas.
Si no está seguro si le aplica lo anterior, consulte a su médico o farmacéutico antes de usar Ryaltris.
Niños y adolescentes
Ryaltris no está recomendado en niños menores de 12 años.
La utilización de Ryaltris durante un periodo de tiempo prolongado puede hacer que los niños y adolescentes crezcan más lentamente. Su médico controlará la altura de su hijo de forma regulary se asegurará de que esté tomando la dosis efectiva más baja posible.
Otros medicamentos y Ryaltris
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Si está tomando otros corticosteroides para la alergia, ya sea por vía oral o mediante inyección, su médico puede aconsejarle que deje de tomarlos cuando empiece a usar Ryaltris.
Si está tomando otros medicamentos por vía oral o usando por vía local (gotas para los ojos o nasales) que contienen olopatadina u otros antihistamínicos, su médico puede recomendarle que deje de tomarlos una vez que comience a usar Ryaltris.
Algunos medicamentos pueden aumentar los efectos de Ryaltris, por lo que su médico le hará controles minuciosos si está tomando estos medicamentos (incluidos algunos para el VIH: ritonavir, cobicistat).
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Ryaltris no debe usarse durante el embarazo a menos que su médico lo considere apropiado.
Si está utilizando Ryaltris, su médico confirmará con usted si debe dar el pecho a su bebé teniendo en cuenta el beneficio para usted gracias al tratamiento y el beneficio de dar el pecho a su bebé. No debe hacer las dos cosas.
Conducción y uso de máquinas
Muy raramente, puede experimentar mareos, letargo, fatiga y somnolencia. Si esto le ocurre, no conduzca ni utilice maquinaria. Tenga en cuenta que beber alcohol puede potenciar estos efectos.
Ryaltris contiene cloruro de benzalconio
Este medicamento contiene 0,02 mg de cloruro de benzalconio en cada pulverización nasal.
El cloruro de benzalconio puede causar irritación o inflamación dentro de la nariz, especialmente cuando se usa durante periodos largos de tratamiento.
3. Cómo usar Ryaltris
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Debe evitarse el contacto con los ojos.
Adultos y adolescentes (12 años de edad y mayores)
La dosis recomendada es de dos pulverizaciones en cada orificio nasalpor la mañana y por la noche.
Uso en niños menores de 12 años de edad
Este medicamento no está recomendado para niños menores de 12 años.
Forma de administración
El pulverizador es para uso nasal.
Lea atentamente las siguientes instrucciones y utilice el pulverizador únicamente según las instrucciones.
Agite el frasco un mínimo de 10 segundos antes de cada uso.
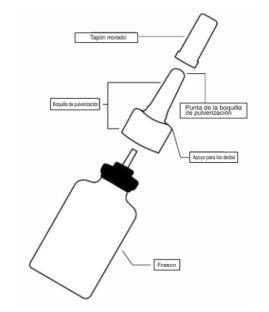
Cuando Ryaltris no esté en uso, el tapón morado debe mantenerse siempre bien colocado en la punta blanca de la boquilla.
Frasco de pulverizador nasal Ryaltris
Preparación del frasco para la pulverización nasal
- Agite el frasco durante un mínimo de 10 segundos y luego retire el tapón morado (ver la figura 1).
- Si está usando el pulverizador por primera vez, debe "cebar" el frasco pulsándolo al aire.
- Sujete el pulverizador nasal firmemente y en posición vertical con los dedos índice y corazón a ambos lados de la boquilla pulverizadora (en los apoyos para los dedos) mientras sujeta la base estriada del frasco con el pulgar.
- Apunte la boquilla lejos de usted y luego presione hacia abajo y suelte la bomba 6 veces hasta que aparezca una fina niebla (vea la figura 2).
- Ahora su bomba está cebada y lista para usar.
- Si no ha usado el pulverizador durante 14 días o más, debe agitar bien el frasco y "volver a cebarlo" pulsando el pulverizador 2 veces o hasta que se produzca una fina niebla.
Cómo utilizar el pulverizador nasal
- Agite el frasco durante un mínimo de 10 segundos antes de cada uso (mañana y noche).
- Suénese la nariz suavemente para limpiar sus orificios nasales.
- Sujete el frasco firmemente con los dedos índice y corazón a ambos lados de la boquilla pulverizadora (en los apoyos para los dedos) mientras sujeta la base estriada del frasco con el pulgar.
- Cierre un orificio nasal con el dedo e inserte con cuidado la punta del pulverizador en el otro orificio nasal, apuntándolo levemente hacia el exterior de la nariz (vea la figura 3).
- Incline la cabeza ligeramente hacia adelante. Presione una vez, de forma rápida y firme, los apoyos para los dedos para activar la bomba.
- Inspire suavemente por la nariz mientras pulveriza. A continuación, espire por la boca (ver la figura 4).
- Repita los pasos anteriores y aplique una segunda pulverización en el mismo orificio nasal.
- Repita con 2 pulverizaciones en el otro orificio nasal.
- Para evitar cualquier obstrucción, después de cada uso, limpie cuidadosamente la boquilla con un pañuelo o paño limpio y seco (ver la figura 5).
- Sujete la boquilla y empuje el tapón morado hacia la base, en la boquilla, hasta que oiga un click (ver la figura 6).


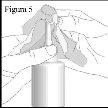

Limpieza de su pulverizador nasal
Si la boquilla se bloquea, haga lo que se describe en los siguientes pasos:
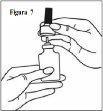
- Retire la boquilla de pulverización suavemente hacia arriba (Ver la figura 7). Retire el tapón morado y coloque solo la boquilla en agua tibia para remojarla.
- No intente desbloquear el pulverizador nasal insertando un alfiler u otro objeto puntiagudo, ya que esto dañará el pulverizador y provocará que no reciba la dosis correcta de medicamento.
- Después de remojar la punta de la boquilla de pulverización durante 15 minutos, enjuague la boquilla de pulverización y el tapón morado con agua tibia y deje que se sequen completamente.
- Vuelva a colocar el tapón morado en la punta de la boquilla pulverizadora y colóquelo de nuevo en el frasco.
- Después de seguir los pasos para limpiar la boquilla de pulverización obstruida, consulte la sección anterior ”Preparación del frasco para la pulverización nasal” y vuelva a cebarlo utilizando 2 pulverizaciones. Vuelva a colocar el tapón morado y su Ryaltris estará listo para su uso.
- Repita los pasos de desbloqueo si es necesario.
Si usa más Ryaltris del que debe
Es poco probable que tenga algún problema, pero si está preocupado o si ha usado dosis superiores a las recomendadas durante un período prolongado, consulte con su médico.
Si utiliza esteroides durante un periodo de tiempo largo o en grandes cantidades puede, en raras ocasiones, afectar a alguna de sus hormonas. En niños puede afectar al crecimiento y desarrollo.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó usar Ryaltris
Si olvidó usar su pulverizador nasal en el momento adecuado, utilícelo tan pronto como lo recuerde, siguiendo posteriormente con el ritmo normal de administración. No use una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Ryaltris
Es muy importante que use su pulverizador nasal regularmente. No interrumpa su tratamiento incluso si se siente mejor a menos que su médico se lo indique.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Pueden ocurrir reacciones inmediatas de hipersensibilidad (alérgicas) después del uso de este medicamento. Estas reacciones pueden ser graves. Debe interrumpir el tratamiento con Ryaltris y buscar ayuda médica inmediatamente si experimenta síntomas como: cara hinchada, lengua o faringe, dificultad para tragar, urticaria, fatiga o dificultad para respirar.
Frecuentes (pueden afectar hasta 1 de cada 10 personas):
- Un sabor amargo en la boca;
- Hemorragia nasal;
- Ligera irritación del interior de la nariz;
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas):
- Mareos;
- Dolores de cabeza;
- Somnolencia;
- Sequedad nasal;
- Boca seca;
- Dolor abdominal;
- Náuseas;
- Fatiga;
Raras (pueden afectar hasta 1 de cada 1.000 personas):
- Vaginosis bacteriana (infección bacteriana de la vagina);
- Ansiedad, depresión, insomnio;
- Letargo, migraña;
- Sequedad de ojos, visión borrosa, molestias en los ojos;
- Dolor de oído;
- Dolor de garganta;
- Estornudos;
- Irritación de garganta;
- Estreñimiento;
- Dolor de lengua;
- Hinchazón y úlceras dentro de la nariz.
Frecuencia no conocida (la frecuencia no puede estimarse a partir de los datos disponibles):
- Aumento de la presión ocular (glaucoma) y/o cataratas que provocan alteraciones visuales;
- Daño en el tabique de la nariz que separa los orificios nasales;
- Dificultad para respirar y/o sibilancias;
- Infección del tracto respiratorio.
Pueden ocurrir efectos adversos sistémicos (efectos secundarios que afectan a todo el cuerpo) cuando este medicamento se usa en dosis altas durante un tiempo prolongado. Es mucho menos probable que ocurran estos efectos si usa un pulverizador nasal de esteroides que si toma esteroides por vía oral. Estos efectos pueden variar según el paciente.
Los esteroides nasales pueden afectar la producción normal de hormonas en su cuerpo, particularmente si usa dosis altas durante mucho tiempo. En niños y adolescentes, este efecto adverso puede hacer que crezcan más lentamente que otros.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Ryaltris
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el frasco y la caja después de “CAD”. La fecha de caducidad es el último día del mes que se indica.No congelar.El pulverizador debe utilizarse dentro de los 2 meses desde su primera administración.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Ryaltris
- Los principios activos son: furoato de mometasona (como monohidrato) y olopatadina (como hidrocloruro). Cada dosis liberada (dosis que sale del pulverizador) contiene furoato de mometasona monohidrato equivalente a 25 microgramos de furoato de mometasona e hidrocloruro de olopatadina equivalente a 600 microgramos de olopatadina.
- Los demás componentes son: celulosa microcristalina (E 460), carmelosa sódica (E 466), fosfato sódico dibásico heptahidratado (E 339), cloruro sódico, cloruro de benzalconio, glicerol, edetato disódico, polisorbato 80 (E 433), ácido clorhídrico (E 507), hidróxido de sodio (E 524) y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Ryaltris es una suspensión blanca y homogénea.
Ryaltris se presenta en un frasco blanco de polietileno de alta densidad con un pulverizador manual de polipropileno, de dosis calibrada. El aplicador nasal está equipado con una tapa violeta de PEAD.
Tamaños de envase:
1 frasco de 20 ml con 56 pulverizaciones
1 frasco de 20 ml con 120 pulverizaciones
1 frasco de 30 ml con 240 pulverizaciones
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Glenmark Pharmaceuticals s.r.o.
Hvezdova 1716/2b
140 78 Praha 4
República Checa
Responsable de la fabricación
Glenmark Pharmaceuticals s.r.o.
Fibichova 143, 566 17 Vysoke Myto
República Checa
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Laboratorios Menarini, S.A.
Alfons XII, 587
08918 Badalona (Barcelona)
España
Este medicamento está autorizado en los estados miembros del EEE bajo los siguientes nombres:
Alemania | Ryaltris 25 Mikrogramm/600 Mikrogramm pro Sprühstoß Nasenspray, Suspension. |
Austria | RYALTRIS 25 Mikrogramm/600 Mikrogramm pro Sprühstoß Nasenspray, Suspension |
Bélgica | RYALTRIS 25 microgrammes/pulvérisation + 600 microgrammes/ pulvérisation, suspension pour pulvérisation nasale RYALTRIS 25 microgram/verstuiving + 600 microgram/verstuiving, neusspray, suspensie RYALTRIS 25 Mikrogramm/ Sprühstö? + 600 Mikrogramm/Sprühstoß Nasenspray, Suspension |
Dinamarca | Ryaltris 25 mikrogram/600 mikrogram pr. dosis Næsespray, suspension |
Eslovaquia | RYALTRIS 25 mikrogramov/600 mikrogramov v jednom vstreknutí nosová suspenzná aerodisperzia |
España | Ryaltris 25 microgramos/600 microgramos/ pulsación, suspensión para pulverización nasal |
Finlandia | RYALTRIS 25 mikrogrammaa + 600 mikrogrammaa / annos nenäsumute, suspensio |
Francia | RYALTRIS 25 microgrammes/600 microgrammes, suspension pour pulvérisation nasale |
Irlanda | Ryaltris 25 microgram/actuation + 600 microgram/actuation nasal spray, suspension |
Italia | RYALTRIS 25 microgrammi/600 microgrammi per erogazione Spray nasale, sospensione |
Noruega | RYALTRIS |
Países Bajos | RYALTRIS 25 microgram/600 microgram, neusspray, suspensie |
Polonia | RYALTRIS |
Reino Unido | RYALTRIS 25 micrograms/600 micrograms per actuation Nasal Spray, suspension |
República Checa | RYALTRIS |
Rumanía | RYALTRIS 25 micrograme/600 micrograme/doza spray nazal suspensie |
Suecia | Ryaltris, 25 mikrogram/ puff + 600 mikrogram/ puff, Nasal spray, suspension |
Fecha de la última revisión de este prospecto:Julio 2024.
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es/).
- País de registro
- Precio medio en farmacia14.75 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a RYALTRIS 25 MICROGRAMOS/600 MICROGRAMOS/PULSACION SUSPENSION PARA PULVERIZACION NASALForma farmacéutica: PRODUCTO USO NASAL, 27,5 µgPrincipio activo: fluticasona furoatoFabricante: Glaxosmithkline (Ireland) LimitedRequiere recetaForma farmacéutica: PRODUCTO USO NASAL, 27,5 MICROGRAMOS/PULVERIZACIÓNPrincipio activo: fluticasona furoatoFabricante: Laboratorios Cinfa S.A.Requiere recetaForma farmacéutica: PRODUCTO USO NASAL, 137 microgramos/50 microgramos/aplicaciónPrincipio activo: fluticasone, combinationsFabricante: Laboratorios Cinfa S.A.Requiere receta
Médicos online para RYALTRIS 25 MICROGRAMOS/600 MICROGRAMOS/PULSACION SUSPENSION PARA PULVERIZACION NASAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de RYALTRIS 25 MICROGRAMOS/600 MICROGRAMOS/PULSACION SUSPENSION PARA PULVERIZACION NASAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











