RINOBANEDIF NASAL POMADE


How to use RINOBANEDIF NASAL POMADE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the user
Rinobanedif Nasal Ointment
Read the entire leaflet carefully before starting to use this medicine, as it contains important information for you.
If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4. |
Contents of the leaflet
- What is Rinobanedif and what is it used for
- What you need to know before starting to use Rinobanedif
- How to use Rinobanedif
- Possible side effects
- Storage of Rinobanedif
- Package contents and additional information
1. What is Rinobanedif and what is it used for
It is a nasal ointment that contains two active antibiotics (bacitracin and neomycin), a corticosteroid, prednisolone (anti-inflammatory), phenylephrine (nasal decongestant), chlorobutanol (antiseptic), and two components with aromatic properties (eucalyptus oil and niaouli essence); when applied to the nasal mucosa, Rinobanedif provides antiseptic, anti-inflammatory, and vasoconstrictive activity.
Antibiotics are used to treat bacterial infections and are not effective against viral infections such as the flu or common cold.
It is essential to follow the instructions regarding dosage, administration interval, and treatment duration indicated by your doctor.
Do not store or reuse this medication. If you have any leftover antibiotic after completing the treatment, return it to the pharmacy for proper disposal. Do not throw away medications down the drain or in the trash.
Rinobanedif is indicated for the local treatment of nasal mucosa conditions that present with congestion, inflammation (such as some symptoms of rhinitis), and/or small wounds, with or without crust formation, in adults and children over 6 years old.
2. What you need to know before starting to use Rinobanedif
Do not use Rinobanedif:
- If you are allergic to the active ingredients or any other component of this medication (listed in section 6).
- If you have tuberculosis, syphilis, fungal infections, or viral infections (such as herpes or chickenpox), including those affecting the respiratory tract.
- In the eyes or on open wounds.
- In children under 6 years old.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Rinobanedif.
- Use with caution in the following situations: coronary artery disease or heart disease, hypertension, irregular heartbeats (arrhythmias), thyroid disease, diabetes mellitus, difficulty urinating due to prostate problems, glaucoma (increased pressure inside the eyes), recent nasal ulcers, recent nasal surgery, or recent nasal trauma.
- Due to the corticosteroid content (prednisolone) via nasal route, any of the side effects reported with the use of oral corticosteroids may occur, especially when used at high doses and for prolonged periods, such as changes in glands located near the kidneys, leading to symptoms like obesity, growth retardation in children, etc. (Cushing's syndrome).
- Do not use the medication for an extended period.
- If you are hypersensitive to certain antibiotics (aminoglycosides), you may be sensitive to neomycin, and if you are sensitive to neomycin, you may be sensitive to bacitracin, components of this medication.
Children
Do not administer to children under 6 years old, as the safety and efficacy of this medication have not been established in this age group.
Children are more prone to experiencing side effects from corticosteroids than adults.
Other medications and Rinobanedif
Inform your doctor or pharmacist if you are taking, have recently taken, or may need to take any other medication, including those purchased without a prescription.
Some medications may increase the effects of Rinobanedif, so your doctor will closely monitor you if you are taking these medications (including some for HIV: ritonavir, cobicistat).
If sufficient internal absorption occurs, it may lead to hypertensive crises and cardiac arrhythmias, especially when administered with MAOIs (antidepressants and other uses), tricyclic antidepressants, and beta-blockers (for the heart). Interactions may also occur with maprotiline (antidepressant) and other aminoglycosides (antibiotics).
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Since some components of Rinobanedif may be absorbed into the circulation, especially with prolonged or excessive administration, it should not be administered during pregnancy, unless the doctor considers that the benefit to the mother outweighs the potential risks to the fetus or baby. Do not apply Rinobanedif during breastfeeding. |
Driving and using machines
No effects on the ability to drive and use machines have been reported.
3. How to use Rinobanedif
Follow the administration instructions for the medication contained in this leaflet or as indicated by your doctor or pharmacist. If you have any doubts, ask your doctor or pharmacist.
The recommended dose is:
Adults and children over 6 years old :Apply 1 to 3 times a day.
The average treatment duration will be 5 days.
Use in children
Do not administer to children under 6 years old.
Nasal route.
Instructions for correct use of the ointment
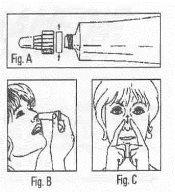
Opening the tube and mode of use: To open the tube, unscrew the cap-nozzle assembly and remove the safety ring (Fig. A). Replace the nozzle, screwing it to the bottom, to pierce the aluminum seal of the tube. Apply a small amount of Rinobanedif to both nostrils (Fig. B), ensuring a uniform distribution of the ointment, for which a gentle external massage is convenient (Fig. C). |
|
After application, clean the end of the tube with a clean, damp cloth. Do not use the container for more than one person.
You should consult your doctor if your symptoms worsen or do not improve after 7 days of treatment.
If you use more Rinobanedif than you should
Due to the form of administration, intoxication is very unlikely.
In case of overdose, the effects that may occur are mainly local irritation, and in case of sufficient absorption of phenylephrine, cardiovascular alterations (cardiac arrhythmias, hypertension) may appear.
In the event of accidental ingestion, if a container is involved, there will be gastric irritation, and there should be no cardiovascular alterations in principle.
Treatment, in case of overdose or accidental ingestion, should include monitoring of blood pressure and electrocardiogram. Additionally, in case of ingestion (equal to or greater than one container), vomiting should be induced or gastric lavage performed, and a mucosal protector administered.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately, or go to a medical center, or call the Toxicology Information Service, Tel. 91 562 04 20, indicating the medication and the amount ingested.
If you forget to use Rinobanedif
Do not use a double dose to make up for the forgotten dose.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
The assessment of side effects is based on the following frequencies:
Very common: may affect more than 1 in 10 people
Common: may affect up to 1 in 10 people
Uncommon: may affect up to 1 in 100 people
Rare: may affect up to 1 in 1,000 people
Very rare: may affect up to 1 in 10,000 people.
Frequency not known (cannot be estimated from available data).
During the use of Rinobanedif ointment, the following side effects may occur:
- Common: allergic contact dermatitis (inflammation at the application site) may occur; after continuous or excessive use, rebound nasal congestion may appear.
- Rare: cutaneous allergic reactions have been observed: redness, itching, dryness, or burning sensation of the nasal mucosa. In rare cases, also: sneezing and signs of internal absorption, such as headache, dizziness, or nervousness, irregular heartbeats, hypertension, and anaphylactic reaction (exaggerated allergic reaction), in general, if the ointment is applied to deep lesions.
- Very rare: ototoxicity (hearing loss), nephrotoxicity (kidney damage), especially if there is renal dysfunction, mild nasal bleeding, nasal dryness, and irritation may occur.
The side effects of corticosteroids increase with factors that increase absorption, such as prolonged use, in extensive areas, or with occlusive materials.
With the use of topical corticosteroids, particularly via nasal route, the following side effects may also occur:
Disorders of smell and taste; rarely, ulceration or perforation of the nasal septum.
Other side effects of corticoids are: skin atrophy, changes in skin color, propensity to bruising, appearance of blood vessels under the skin surface, folliculitis (inflammation of hair follicles), increased hair growth, stretch marks, acne, secondary infections such as fungal infections.
Side effects due to corticosteroid absorption: Cushing's syndrome, characterized by obesity, rounded face, fat accumulation in the cervical area, delayed wound healing, psychiatric symptoms, etc.; cataracts, glaucoma.
Reporting side effects
If you experience any type of side effect, consult your doctor or pharmacist, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medications: https://www.notificaRAM.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Rinobanedif
No special storage conditions are required.
This medication should be used within 6 months after opening.
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date shown on the container after CAD. The expiration date is the last day of the month indicated.
Medications should not be thrown away down the drain or in the trash. Deposit the containers and medications you no longer need at the SIGRE collection point in the pharmacy. If you have any doubts, ask your pharmacist how to dispose of the containers and medications you no longer need. This will help protect the environment.
6. Package contents and additional information
Rinobanedif composition
- The active ingredients are: zinc bacitracin, neomycin sulfate, prednisolone, phenylephrine hydrochloride, chlorobutanol hemihydrate, eucalyptus oil, and niaouli essence. Each gram of ointment contains: 500 UI of zinc bacitracin, 5 mg of neomycin sulfate (equivalent to 3.5 mg of neomycin base), 3 mg of prednisolone (0.3%), 2.5 mg of phenylephrine hydrochloride (0.25%), 8 mg of chlorobutanol hemihydrate, 2.00 mg - 2.86 mg of eucalyptus oil (equivalent to 2 mg of 1,8-cineole) and 1.54 mg - 2.22 mg of niaouli essence (equivalent to 1 mg of 1,8-cineole).
- The other components (excipients) are: cholesterol, plastibase, white petrolatum, and liquid petrolatum.
Product appearance and package contents
Rinobanedif is a nasal ointment; it is greasy, with a yellowish-white color.
Each container holds a 10 g aluminum tube of nasal ointment.
Marketing authorization holder and manufacturer
Marketing authorization holder:
TEOFARMA S.R.L.
Via F.lli Cervi, 8
27010 Valle Salimbene (PV) - Italy
Manufacturer:
DOPPEL FARMACEUTICI, S.R.L.
Via Martiri delle Foibe, 1. Cortemaggiore
(Piacenza)- 29016- Italy
Date of the last revision of this leaflet:February 2020
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RINOBANEDIF NASAL POMADEDosage form: NASAL PRODUCT, 27.5 µgActive substance: fluticasone furoateManufacturer: Glaxosmithkline (Ireland) LimitedPrescription requiredDosage form: NASAL PRODUCT, 27.5 MICROGRAMS/SPRAYActive substance: fluticasone furoateManufacturer: Laboratorios Cinfa S.A.Prescription requiredDosage form: NASAL PRODUCT, 137 micrograms/50 micrograms/applicationActive substance: fluticasone, combinationsManufacturer: Laboratorios Cinfa S.A.Prescription required
Online doctors for RINOBANEDIF NASAL POMADE
Discuss questions about RINOBANEDIF NASAL POMADE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions