
OCREVUS 300 MG CONCENTRADO PARA SOLUCION PARA PERFUSION

Cómo usar OCREVUS 300 MG CONCENTRADO PARA SOLUCION PARA PERFUSION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Ocrevus 300mg concentrado para solución para perfusión
ocrelizumab
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico.
- Si experimenta efectos adversos, consulte a su médico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Ocrevus y para qué se utiliza
- Qué necesita saber antes de empezar a usar Ocrevus
- Cómo usar Ocrevus
- Posibles efectos adversos
- Conservación de Ocrevus
- Contenido del envase e información adicional
1. Qué es Ocrevus y para qué se utiliza
Qué es Ocrevus
Ocrevus contiene el principio activo “ocrelizumab”. Se trata de un tipo de proteína llamada “anticuerpo monoclonal”. Los anticuerpos actúan uniéndose a dianas específicas en su organismo.
Para qué se utiliza Ocrevus
Ocrevus se utiliza para tratar adultos con:
- Formas recurrentes de esclerosis múltiple (EMR)
- Esclerosis múltiple primaria progresiva (EMPP) temprana
Qué es la Esclerosis Múltiple:
La esclerosis múltiple (EM) afecta al sistema nervioso central, especialmente a los nervios del cerebro y a la médula espinal. En la EM, el sistema inmunitario (sistema de defensa del organismo) funciona de forma incorrecta, atacando la capa protectora (llamada vaina de mielina) situada alrededor de las células nerviosas y provocando inflamación. La ruptura de la vaina de mielina impide a los nervios funcionar adecuadamente.
Los síntomas de EM dependen de la parte del sistema nervioso central que esté afectada y pueden incluir, problemas para caminar y mantener el equilibrio, debilidad, entumecimiento, visión doble y borrosa, mala coordinación y problemas de vejiga.
- En las formas recurrentes de EMlos pacientes presentan crisis repetidas de síntomas (brotes). Los síntomas pueden aparecer de forma repentina en el plazo de unas pocas horas, o lentamente en el transcurso de varios días. Los síntomas desaparecen o mejoran entre cada brote pero el daño puede acumularse y producir una discapacidad permanente.
- Enlos pacientes con EM primaria progresivageneralmente los síntomas empeoran de forma continuada desde el inicio de la enfermedad.
Cómo funciona Ocrevus
Ocrevus se une a linfocitos B específicos, que son un tipo de glóbulos blancos que forman parte del sistema inmunitario y desempeñan una función en la EM. Ocrevus se une y elimina estos linfocitos B específicos. Esto reduce la inflamación y los ataques sobre la vaina de mielina, reduce las probabilidades de experimentar una recaída y ralentiza la progresión de la enfermedad.
- En las formas recurrentes de EM (EMR), Ocrevus ayuda a reducir de forma significativa el número de crisis (brotes) y ralentizar de forma significativa la progresión de la enfermedad. Ocrevus también aumenta de forma significativa la probabilidad de que un paciente no presente evidencia de actividad de la enfermedad (lesiones cerebrales, brotes y empeoramiento de la discapacidad).
- En la EM primaria progresiva (EMPP), Ocrevus ayuda a ralentizar la progresión de la enfermedad y a reducir el deterioro de la velocidad de la marcha.
2. Qué necesita saber antes de empezar a usar Ocrevus
No use Ocrevus:
- si es alérgico al ocrelizumab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si actualmente presenta una infección.
- si le han diagnosticado problemas graves en su sistema inmunitario.
- si tiene cáncer.
Si usted no está seguro, hable con su médico antes de usar Ocrevus.
Advertencias y precauciones
Consulte a su médico antes de empezar a usar Ocrevussi alguna de las siguientes condiciones se aplica a su caso. Puede que su médico decida retrasar su tratamiento con Ocrevus o que decida que usted no puede usar Ocrevus si:
- usted tiene una infección. Su médico esperará hasta que la infección se haya resuelto antes de administrarle Ocrevus.
- usted ha padecido alguna vez hepatitis Bo es portador del virus de la hepatitis B. Esto se debe a que medicamentos como Ocrevus pueden hacer que el virus de la hepatitis B vuelva a activarse. Antes del tratamiento con Ocrevus, su médico comprobará si está en riesgo de infección por hepatitis B. Los pacientes que han tenido hepatitis B o que son portadores del virus de la hepatitis B se someterán a un análisis de sangre y serán supervisados por un médico para detectar signos de infección por hepatitis B.
- usted padece cáncero si ha padecido cáncer en el pasado. Es posible que su médico opte por retrasar su tratamiento con Ocrevus.
Efectos en el sistema inmunitario:
- Enfermedades que afectan a su sistema inmunitario:si usted padece otra enfermedad que afecta al sistema inmunitario. Es posible que no sea apto para el tratamiento con Ocrevus.
- Medicamentos que afectan a su sistema inmunitario:si usted ha tomado alguna vez, está tomando o tiene previsto tomar medicamentos que afectan al sistema inmunitario – como quimioterapia, inmunosupresores u otros medicamentos empleados para tratar la EM. Es posible que su médico opte por retrasar su tratamiento con Ocrevus o que le pida que interrumpa dichos medicamentos antes de comenzar el tratamiento con Ocrevus. Para más información ver más adelante “Otros medicamentos y Ocrevus”.
Reacciones relacionadas con la perfusión
- Las reacciones relacionadas con la perfusión son el efecto adverso más frecuente del tratamiento con Ocrevus.
- Informe inmediatamente a su médico o enfermero si tiene cualquier reacción relacionada con la perfusión(ver en la sección 4 una lista de reacciones relacionadas con la perfusión). Las reacciones relacionadas con la perfusión pueden producirse durante la perfusión o hasta 24 horas después de la misma.
- Para reducir el riesgo de reacciones relacionadas con la perfusión, su médico le administrará otros medicamentos antes de cada perfusión de Ocrevus (ver sección 3) y se le someterá a una estrecha supervisión durante la perfusión y al menos una hora después de la administración de la misma.
Infecciones
- Informe a su médico antes de recibir el tratamiento con Ocrevus si cree que podría tener una infección. Su médico esperará a que su infección se haya resuelto antes de administrarle el tratamiento con Ocrevus.
- Usted podría contraer infecciones más fácilmente con Ocrevus. Esto se debe a que las células inmunitarias sobre las que actúa Ocrevus también ayudan a combatir infecciones.
- Antes de que usted comience el tratamiento con Ocrevus y antes de las siguientes perfusiones, su médico puede solicitarle un análisis de sangre para confirmar el estado de su sistema inmunitario ya que las infecciones pueden presentarse más frecuentemente en caso de problemas graves en su sistema inmunitario.
- Si usted ha sido tratado con Ocrevus para esclerosis múltiple primaria progresiva, y tiene dificultades al tragar, Ocrevus puede aumentar el riesgo de neumonía grave.
- Informe inmediatamente a su médico o enfermero si experimenta cualquiera de estos signos de infección durante o después del tratamiento con Ocrevus:
- fiebre o escalofríos
- tos que no desaparece
- herpes (como aftas, herpes zóster o úlceras genitales).
- Informe inmediatamente a su médico o enfermero si cree que la EM está empeorando o si percibe cualquier síntoma nuevo. Esto se debe a una infección muy rara y potencialmente mortal del cerebro, llamada “leucoencefalopatía multifocal progresiva” (LMP), que puede causar síntomas similares a los de la EM. La LMP puede presentarse en pacientes que toman Ocrevus.
Informe a su pareja o cuidadoracerca del tratamiento con Ocrevus. Ellos podrían percibir síntomas de LMP que usted no percibe, como fallos de memoria, problemas para pensar, dificultad para caminar, pérdida de visión o cambios en su forma de hablar. Es posible que su médico necesite estudiarlos.
Vacunas
- Informe a su médico si ha recibido recientemente cualquier vacuna o podría recibir una vacuna en el futuro próximo.
- Durante el tratamiento con Ocrevus, usted no debe recibir vacunas vivas o vivas atenuadas (por ejemplo, BCG para la tuberculosis o vacunas contra la fiebre amarilla).
- Su médico puede recomendarle que se vacune de la gripe estacional.
- Su médico verificará si usted necesita alguna vacuna antes de comenzar el tratamiento con Ocrevus. Las vacunas deben administrarse al menos 6 semanas antes de comenzar el tratamiento con Ocrevus.
Niños y adolescentes
Ocrevus no está destinado para su uso en niños y adolescentes menores de 18 años de edad. Esto se debe a que aún no se ha estudiado en este grupo de edad.
Otros medicamentos y Ocrevus
Informe a su médico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Especialmente, informe a su médico si:
- usted ha tomado alguna vez, está tomando o tiene previsto tomar medicamentos que afectan el sistema inmunitario– como quimioterapia, inmunosupresores u otros medicamentos empleados para tratar la EM. El efecto sobre el sistema inmunitario de estos medicamentos administrados junto con Ocrevus podría ser demasiado fuerte. Es posible que su médico opte por retrasar su tratamiento con Ocrevus o que le pida que interrumpa dichos medicamentos antes de comenzar el tratamiento con Ocrevus.
- usted está tomando medicamentos para la hipertensión arterial. Esto se debe a que Ocrevus puede disminuir su tensión arterial. Puede que su médico le pida que interrumpa sus medicamentos para la tensión arterial 12 horas antes de cada perfusión de Ocrevus.
Si alguna de estas condiciones se aplica a su caso (o usted no está seguro), hable con su médico antes de usar Ocrevus.
Embarazo
- Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de que se le administre este medicamento. Esto se debe a que Ocrevus puede cruzar la barrera placentaria y afectar a su bebé.
- No use Ocrevus si está embarazada a menos que lo haya comentado con su médico. Su médico sopesará el beneficio de usar Ocrevus frente al riesgo que esto constituye para su bebé.
- Consulte a su médico antes de vacunar a su bebé.
Anticoncepción para mujeres
Si usted puede quedarse embarazada (concebir), las mujeres en edad fértil, deberán utilizar métodos anticonceptivos:
- durante el tratamiento con Ocrevus y
- durante 4 meses después de la última perfusión de Ocrevus.
Lactancia
No dé el pecho mientras recibe tratamiento con Ocrevus. Esto se debe a que Ocrevus puede pasar a la leche materna.
Conducción y uso de máquinas
Se desconoce si Ocrevus puede afectar a su capacidad para conducir o utilizar herramientas o máquinas.
Su médico le comunicará si la EM puede afectar a su capacidad para conducir o utilizar herramientas o máquinas con seguridad.
Ocrevus contiene sodio
Este medicamento contiene menos de 1 mmol de sodio(23 mg) por dosis, esto es, esencialmente “exento de sodio”.
3. Cómo usar Ocrevus
Ocrevus será administrado por un médico o enfermero experimentado en el uso de este tratamiento.
Le mantendrá en observación durante la administración del medicamento por si sufre algún efecto adverso. Ocrevus siempre se le administrará como goteo (perfusión intravenosa).
Medicamentos que recibirá antes de Ocrevus
Antes de recibir Ocrevus, se le administrarán otros medicamentos para prevenir o reducir posibles efectos adversos como reacciones relacionadas con la perfusión (ver las secciones 2 y 4 para más información sobre las reacciones relacionadas con la perfusión).
Recibirá un corticosteroide y un antihistamínico antes de cada perfusión y puede que también se le administren medicamentos para reducir la fiebre.
Cuánto Ocrevus y con qué frecuencia se administra
Usted recibirá una dosis total de 600 mg de Ocrevus cada 6 meses.
- La primera dosis de 600 mg de Ocrevus se administrará como 2 perfusiones (300 mg cada una) separadas por un intervalo de 2 semanas. Cada perfusión durará alrededor de 2 horas y 30 minutos.
- Las siguientes dosis de 600 mg de Ocrevus se administrarán como una sola perfusión. Dependiendo de la velocidad de la siguiente perfusión, ésta durará bien alrededor de 3 horas y 30 minutos o bien 2 horas.
Cómo administrar Ocrevus
- Ocrevus será administrado por un médico o un enfermero. Se administrará como una perfusión en una vena (perfusión intravenosa o perfusión “IV”).
- Será supervisado estrechamente mientras recibe Ocrevus y durante al menos 1 hora después de la perfusión. Esto es por si presenta algún efecto adverso como reacciones relacionadas con la perfusión. La perfusión podrá ralentizarse, interrumpirse temporalmente o suspenderse permanentemente en caso de que presente una reacción relacionada con la perfusión, y dependiendo de la gravedad de la misma (ver las secciones 2 y 4 para más información sobre las reacciones relacionadas con la perfusión).
Si se olvida la administración de una perfusión de Ocrevus
- Si se salta una perfusión de Ocrevus, hable con su médico para programar una nueva perfusión lo antes posible. No espere hasta su siguiente perfusión programada.
- Para obtener el beneficio completo de Ocrevus, es importante que reciba cada perfusión cuando corresponda.
Si interrumpe el tratamiento con Ocrevus
- Es importante que continúe su tratamiento durante el tiempo que usted y su médico consideren que le está ayudando.
- Algunos efectos adversos pueden estar relacionados con niveles bajos de linfocitos B. Después de finalizar el tratamiento con Ocrevus, puede continuar experimentando efectos adversos hasta que sus linfocitos B vuelvan a alcanzar los niveles normales. Sus niveles en sangre de linfocitos B aumentarán gradualmente hasta alcanzar niveles normales. Esto puede prolongarse durante un periodo de entre seis meses y dos años y medio, o incluso más años en casos raros.
- Antes de empezar a tomar cualquier otro medicamento, informe a su médico sobre cuándo recibió la última perfusión de Ocrevus.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Se han comunicado los siguientes efectos adversos con Ocrevus:
Efectos adversos graves:
Reacciones relacionadas con la perfusión
- Las reacciones relacionadas con la perfusión son el efecto adverso más frecuente del tratamiento con Ocrevus (muy frecuentes: pueden afectar a más de 1 de cada 10 personas). En la mayoría de los casos son reacciones leves, pero pueden producirse algunas reacciones graves.
- Informe inmediatamente a su médico o enfermero si experimenta cualquier signo o síntoma de una reacción relacionada con la perfusión durante la perfusión o hasta 24 horas después de la perfusión.Los síntomas pueden incluir, entre otros:
- picor de la piel
- erupción cutánea
- ronchas
- enrojecimiento de la piel
- irritación o dolor de garganta
- dificultad para respirar
- hinchazón de la garganta
- rubor
- tensión arterial baja
- fiebre
- cansancio
- dolor de cabeza
- mareo
- náuseas
- latido cardíaco rápido.
- Si presenta una reacción relacionada con la perfusión, se le administrarán medicamentos para tratarla, y es posible que la perfusión tenga que ralentizarse o suspenderse. Cuando la reacción haya remitido se podrá continuar con la perfusión. Si la reacción relacionada con la perfusión es potencialmente mortal, su médico suspenderá permanentemente el tratamiento con Ocrevus.
Infecciones
- Usted podría contraer infecciones más fácilmente con Ocrevus. Se han observado las siguientes infecciones en pacientes tratados con Ocrevus en el contexto de la EM:
- Muy frecuentes: pueden afectar a más de 1 de cada 10 personas
- dolor de garganta y secreción nasal (infección de las vías respiratorias superiores)
- gripe.
- Frecuentes: pueden afectar hasta 1 de cada 10 personas
- infección sinusal
- bronquitis (inflamación del tubo bronquial)
- infección por herpes (afta o herpes zóster)
- infección de estómago e intestino (gastroenteritis)
- infección de las vías respiratorias
- infección vírica
- infección cutánea (celulitis)
Algunas de ellas pueden ser graves.
- Informe inmediatamente a su médico o enfermero si experimenta cualquiera de estos signos de infección:
- fiebre o escalofríos
- tos que no desaparece
- herpes (como aftas, herpes zóster y úlceras genitales).
Otros efectos adversos
Muy frecuentes:pueden afectar a más de 1 de cada 10 personas
- disminución de ciertas proteínas en la sangre (inmunoglobulinas) que ayudan a proteger frente a infecciones.
Frecuentes:pueden afectar hasta 1 de cada 10 personas
- secreción del ojo con picor, enrojecimiento e hinchazón (conjuntivitis)
- tos
- acumulación de moco denso en la nariz, la garganta o el pecho
- niveles bajos de un tipo de glóbulos blancos (neutropenia).
Desconocida: no se sabe con que frecuencia se producen estos efectos adversos
- reducción de los globulos blancos que se produce con retraso
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Ocrevus
Los profesionales sanitarios del hospital o la clínica conservarán Ocrevus bajo las siguientes condiciones:
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- No utilice este medicamento después de la fecha de caducidad que aparece en el envase exterior y en la etiqueta del vial después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
- Conservar este medicamento en nevera (2oC - 8oC). No congelar. Conservar los viales en el envase exterior para protegerlos de la luz.
Ocrevus debe diluirse antes de su administración. La dilución la realizará un profesional sanitario. Se recomienda utilizar el medicamento inmediatamente después de la dilución. Si no se utiliza inmediatamente, los periodos de conservación durante el uso y las condiciones antes de su uso serán responsabilidad del profesional sanitario y, por lo general, no superarán las 24 horas a 2ºC – 8ºC y las 8 horas posteriores a temperatura ambiente.
Los medicamentos no se deben tirar por los desagües. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Ocrevus
- El principio activo es ocrelizumab. Cada vial contiene 300 mg de ocrelizumab en 10 ml a una concentración de 30 mg/ml.
- Los demás componentes son acetato de sodio trihidrato (ver Sección 2 “Ocrevus contiene sodio”), ácido acético glacial, trehalosa dihidrato, polisorbato 20 y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
- Ocrevus es una solución entre transparente y ligeramente opalescente, y entre incolora y marrón claro.
- Se suministra como concentrado para solución para perfusión.
- Este medicamento está disponible en envases que contienen 1 ó 2 viales (viales de 10 ml de concentrado). Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Roche Registration GmbH
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Alemania
Responsable de la fabricación
Roche Pharma AG
Emil-Barell-Strasse 1
D-79639 Grenzach-Wyhlen
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien N.V. Roche S.A. Tél/Tel: +32 (0) 2 525 82 11 | Lietuva UAB “Roche Lietuva” Tel.: +370 5 2546799 |
| Luxembourg/Luxemburg (Voir/siehe Belgique/Belgien) |
Ceská republika Roche s. r. o. Tel.: +420 - 2 20382111 | Magyarország Roche (Magyarország) Kft. Tel.: +36 - 1 279 4500 |
Danmark Roche Pharmaceuticals A/S Tel.: +45 - 36 39 99 99 | Malta (See Ireland) |
Deutschland Roche Pharma AG Tel.: +49 (0) 7624 140 | Nederland Roche Nederland B.V. Tel.: +31 (0) 348 438050 |
Eesti Roche Eesti OÜ Tel.: + 372 - 6 177 380 | Norge Roche Norge AS Tel.: +47 - 22 78 90 00 |
Ελλ?δα Roche (Hellas) A.E. Tel.: +30 210 61 66 100 | Österreich Roche Austria GmbH Tel.: +43 (0) 1 27739 |
España Roche Farma S.A. Tel: +34 - 91 324 81 00 | Polska Roche Polska Sp.z o.o. Tel.: +48 - 22 345 18 88 |
France Roche Tel: +33 (0) 1 47 61 40 00 | Portugal Roche Farmacêutica Química, Lda Tel.: +351 - 21 425 70 00 |
Hrvatska Roche d.o.o. Tel.: +385 1 4722 333 | România Roche România S.R.L. Tel.: +40 21 206 47 01 |
Ireland Roche Products (Ireland) Ltd. Tel.: +353 (0) 1 469 0700 | Slovenija Roche farmacevtska družba d.o.o. Tel.: +386 - 1 360 26 00 |
Ísland Roche Pharmaceuticals A/S c/o Icepharma hf Sími: +354 540 8000 | Slovenská republika Roche Slovensko, s.r.o. Tel.: +421 - 2 52638201 |
Italia Roche S.p.A. Tel.: +39 - 039 2471 | Suomi/Finland Roche Oy Puh/Tel: +358 (0) 10 554 500 |
K?προς Γ.Α.Σταμ?της & Σια Λτδ. Tel.: +357 - 22 76 62 76 | Sverige Roche AB Tel.: +46 (0) 8 726 1200 |
Latvija Roche Latvija SIA Tel.: +371 - 6 7039831 | United Kingdom (Northern Ireland) Roche Products (Ireland) Ltd. Tel: +44 (0) 1707 366000 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.Esta información está destinada únicamente a profesionales sanitarios:
Para más información lea la Ficha Técnica.
Para mejorar la trazabilidad de los medicamentos biológicos, se debe registrar claramente el nombre y el número de lote del producto administrado.
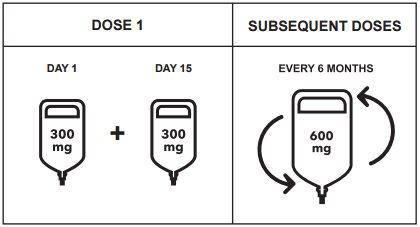
Posología
- Dosis inicial
La dosis inicial de 600 mg se administra como dos perfusiones intravenosas separadas; primero una perfusión de 300 mg seguida de una segunda perfusión de 300 mg que se administra 2 semanas después.
- Dosis posteriores
Después de la dosis inicial, las dosis posteriores de ocrelizumab se administran como una única perfusión intravenosa de 600 mg cada 6 meses (Tabla 1). La primera de las dosis posteriores de 600 mg se debe administrar seis meses después de la primera perfusión de la dosis inicial. Debe respetarse un intervalo mínimo de 5 meses entre cada dosis de ocrelizumab.
Figura 1: Dosis y pauta posológica de Ocrevus
Manejo de las RRP antes de la perfusión
- El tratamiento con ocrelizumab debe iniciarlo y supervisarlo un profesional sanitario experimentado y con acceso a un apoyo médico adecuado para el manejo de reacciones graves como las reacciones graves relacionadas con la perfusión (RRP), reacciones de hipersensibilidad y/o reacciones anafilácticas.
- Premedicación para las RRP
Se administrarán las dos siguientes premedicaciones antes de cada perfusión de Ocrevus para reducir la frecuencia y la gravedad de las RRP:
- 100 mg de metilprednisolona intravenosa (o equivalente) aproximadamente 30 minutos antes de cada perfusión;
- un antihistamínico aproximadamente 30-60 minutos antes de cada perfusión.
Además, también podrá considerarse la premedicación con un antipirético (p. ej., paracetamol) aproximadamente 30-60 minutos antes de cada perfusión de Ocrevus.
- Puede producirse hipotensión, como síntoma de una RRP, durante las perfusiones de Ocrevus. Por lo tanto, debe considerarse la interrupción de tratamientos antihipertensivos durante 12 horas antes de y durante cada perfusión de Ocrevus. No se han estudiado los pacientes con antecedentes de insuficiencia cardíaca congestiva (clases III y IV de la New York Heart Association).
Instrucciones para la dilución
- El producto debe ser preparado por un profesional sanitario mediante técnicas asépticas. No agitar el vial. Se debe utilizar una aguja y una jeringa estériles para preparar la solución para perfusión diluida.
- El medicamento está destinado para un solo uso.
- El concentrado puede contener partículas finas translúcidas y/o reflectivas asociadas con opalescencia aumentada. No utilizar El concentrado si presenta cambio de coloración o si el concentradocontiene alguna partícula extraña.
- El medicamento se debe diluir antes de su administración. Las soluciones para administración intravenosa se preparan por dilución del concentrado en una bolsa de perfusión que contiene 9 mg/ml de solución para perfusión de cloruro de sodio isotónico al 0,9% (300 mg/250 ml ó 600 mg/500 ml), para alcanzar una concentración final de aproximadamente 1,2 mg/ml.? La solución para perfusión diluida debe administrarse mediante un equipo de perfusión con un filtro en línea de 0,2 o 0,22 micras.
- Antes de iniciar la perfusión intravenosa, el contenido de la bolsa de perfusión debe estar a temperatura ambiente para evitar una reacción a la perfusión debida a la administración de la solución a temperaturas bajas.
Forma de administración
- Después de la dilución, el tratamiento se administra como perfusión intravenosa a través de una vía específica.
- Las perfusiones de no se deben administrar en perfusión intravenosa rápida o bolo.
Tabla 1: Dosis y pauta posológica de Ocrevus
Cantidad de ocrelizumab a administrar | Instrucciones de perfusión | ||
Dosis inicial (600 mg) dividida en 2 perfusiones | Perfusión 1 | 300 mg en 250 ml |
|
Perfusión 2 (2 semanas después) | 300 mg en 250 ml | ||
Dosis posteriores (600 mg) perfusión únicauna vez cada 6 meses | Opción 1 Perfusión de aproximadamente 3,5 horas de duración | 600 mg en 500 ml |
|
O | |||
Opción 2 Perfusión de aproximadamente 2 horas de duración | 600 mg en 500 ml |
|
Manejo de las RRP durante y después de la perfusión
Se debe vigilar a los pacientes durante la perfusión y durante al menos una hora tras finalizar la misma.
Durante la perfusión
- Ajustes de la perfusión en caso de RRP
En caso de que se produzcan RRP durante la perfusión, consulte los siguientes ajustes.
RRP potencialmente mortales
Si hay signos de una RRP potencialmente mortal o incapacitante durante una perfusión, tales como hipersensibilidad aguda o síndrome de insuficiencia respiratoria aguda, la perfusión debe suspenderse inmediatamente y el paciente recibirá tratamiento adecuado. En estos pacientes la perfusión se debe suspender de forma permanente (ver sección 4.3).
RRP graves
Si un paciente experimenta una RRP grave (como disnea) o una combinación de síntomas de rubor, fiebre y dolor de garganta, la perfusión se interrumpirá inmediatamente y el paciente recibirá tratamiento sintomático. La perfusión se reiniciará únicamente después de la resolución de todos los síntomas. La velocidad de perfusión inicial en el momento del reinicio debe ser la mitad de la velocidad de perfusión en el momento de aparición de la reacción. No se requiere ningún ajuste de la perfusión para las perfusiones posteriores, a menos que el paciente experimente una RRP.
RRP de leves a moderadas
Si un paciente experimenta una RRP de leve a moderada (p. ej., cefalea), la velocidad de perfusión debe reducirse a la mitad de la velocidad de perfusión en el momento de la aparición del acontecimiento. Esta velocidad reducida se debe mantener durante al menos 30 minutos. Si se tolera, la velocidad de perfusión podrá incrementarse en función de la velocidad de perfusión inicial del paciente. No se requiere ningún ajuste de la perfusión para las perfusiones posteriores, a menos que el paciente experimente una RRP.
- Los pacientes que experimenten síntomas pulmonares graves, como broncoespasmo o exacerbación asmática, deben interrumpir la perfusión inmediatamente y de forma permanente. Tras administrar el tratamiento sintomático, se supervisará al paciente hasta la resolución de los síntomas pulmonares, ya que la mejoría inicial de los síntomas clínicos podría ir seguida de un deterioro.
- Puede ser clinicamente indistinguible la hipersensibilidad de una RRP en términos de síntomas. Si se sospecha una reacción de hipersensibilidad durante la perfusión, la perfusión se debe suspender inmediatamente y de forma permanente.
Después de la perfusión
- Se debe supervisar a los pacientes durante al menos una hora después de la finalización de la perfusión para detectar posibles síntomas de una RRP.
- Los médicos deben advertir a los pacientes de que una RRP puede producirse dentro de las 24 horas posteriores a la perfusión.
Periodo de Validez
Vial sin abrir
2 años
Solución diluida para perfusión intravenosa
- Se ha demostrado la estabilidad química y física durante 24 horas a 2-8º C y posteriormente 8 horas a temperatura ambiente.
- Desde el punto de vista microbiológico, la perfusión preparada debe usarse inmediatamente. Si no se usa inmediatamente, los periodos de conservación y las condiciones antes de su utilización serán responsabilidad del usuario y, por lo general, no superarán las 24 horas a 2-8ºC y posteriormente 8 horas a temperatura ambiente, a menos que la dilución se lleve a cabo en condiciones asépticas controladas y validadas.
- En caso de que una perfusión intravenosa no pueda completarse en el mismo día, la solución restante debe descartarse.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a OCREVUS 300 MG CONCENTRADO PARA SOLUCION PARA PERFUSIONForma farmacéutica: INYECTABLE PERFUSION, 120 mg (80 mg/kg) belimumabPrincipio activo: BelimumabFabricante: Glaxosmithkline (Ireland) LimitedRequiere recetaForma farmacéutica: INYECTABLE, 200 mgPrincipio activo: BelimumabFabricante: Glaxosmithkline (Ireland) LimitedRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, 400 mg (80 mg/kg) belimumabPrincipio activo: BelimumabFabricante: Glaxosmithkline (Ireland) LimitedRequiere receta
Médicos online para OCREVUS 300 MG CONCENTRADO PARA SOLUCION PARA PERFUSION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de OCREVUS 300 MG CONCENTRADO PARA SOLUCION PARA PERFUSION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes







