NEPEXTO 50 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA

Cómo usar NEPEXTO 50 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Nepexto 50 mg solución inyectable en pluma precargada
etanercept
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Su médico además le dará una Tarjeta para el Paciente, la cual contiene información de seguridad importante que usted necesita conocer antes y durante el tratamiento con Nepexto.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado a usted o a un niño que está a su cargo y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted o el niño a su cargo, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Nepexto y para qué se utiliza
- Qué necesita saber antes de empezar a usar Nepexto
- Cómo usar Nepexto
- Posibles efectos adversos
- Conservación de Nepexto
- Contenido del envase e información adicional
- Instrucciones de uso
1. Qué es Nepexto y para qué se utiliza
Nepexto contiene el principio activo etanercept.
Nepexto es un medicamento que se fabrica a partir de dos proteínas humanas. Bloquea la actividad de otra proteína, que se encuentra en el organismo, que produce inflamación. Este medicamento actúa reduciendo la inflamación asociada a ciertas enfermedades.
Se puede usar Nepexto en adultos (a partir de los 18 años) para:
- Artritis reumatoide(trastorno autoinmunitario que afecta principalmente a las articulaciones) de moderada a grave.
- Artritis psoriásica(un tipo de artritis inflamatoria que puede afectar a cualquier articulación del cuerpo).
- Espondiloartritis axial grave(un tipo de artritis inflamatoria crónica que afecta a la columna vertebral o a las articulaciones sacroilíacas) incluyendo espondilitis anquilosante(un tipo de artritis que afecta a la columna vertebral).
- Psoriasis(áreas abultadas, rojas y escamosas en la piel) de leve a moderada.
En caso de que se use Nepexto, generalmente cuando otros tratamientos más comúnmente utilizados no han funcionado adecuadamente, o bien dichos tratamientos no son los adecuados para ellos.
En el tratamiento de la artritis reumatoide, este medicamento se utiliza normalmente en combinación con metotrexato, aunque también puede utilizarse como único medicamento, en el caso de que el tratamiento con metotrexato no sea apropiado para usted. Nepexto puede ralentizar el daño causado por la artritis reumatoide en sus articulaciones y mejorar su capacidad para realizar las actividades diarias, tanto si se utiliza solo o en combinación con metotrexato.
En el caso de los pacientes que presentan artritis psoriásicacon afectación múltiple de las articulaciones, este medicamento puede mejorar su capacidad para realizar las actividades normales diarias.
En el caso de los pacientes que presentan articulaciones simétricas múltiples, hinchadas o dolorosas(por ejemplo, manos, muñecas y pies), este medicamento puede retrasar el progreso del daño estructural de dichas articulaciones causado por la enfermedad.
Nepexto está también indicado para el tratamiento en niños y adolescentes con las siguientes enfermedades:
- Para los siguientes tipos de artritis idiopática juvenil cuando el tratamiento con metotrexato no ha funcionado adecuadamente, o bien no es el adecuado para ellos:
- Poliartritis (con factor reumatoide positivo o negativo) y oligoartritis extendida en pacientes a partir de 2 años de edad y 62,5 kg de peso.
- Artritis psoriásica en pacientes a partir de 12 años y 62,5 kg de peso.
- Para la artritis relacionada con entesitis en pacientes a partir de 12 años de edad y 62,5 kg de peso para los que el uso de otros tratamientos más comúnmente utilizados no han funcionado adecuadamente, o bien dichos tratamientos no son los adecuados para ellos.
- Psoriasis grave en pacientes a partir de 6 años de edad y 62,5 kg de peso que han tenido una respuesta inadecuada a (o son incapaces de tomar) fototerapias u otras terapias sistémicas.
2. Qué necesita saber antes de empezar a usar Nepexto
No use Nepexto
- Si usted o el niño que está a su cuidado son alérgicos a etanercepto a cualquiera de los demás componentes de Nepexto(incluidos en la sección 6). Si usted o el niño experimentan reacciones alérgicas, tales como opresión torácica, respiración jadeante, vértigo o erupción, no inyecte más Nepexto y póngase inmediatamente en contacto con su médico.
- Si usted o el niño padecen o tienen riesgo de desarrollar una infección grave de la sangre
- denominada sepsis. Si no está seguro, consulte a su médico.
- Si usted o el niño padecen una infección de cualquier tipo. Si no está seguro, consulte a su médico.
Advertencias y precauciones
Consulte a su médico antes de empezar a usar Nepexto.
- Reacciones alérgicas: Si usted o el niño experimentan reacciones alérgicas, tales como opresión torácica, respiración jadeante, vértigo o erupción, deje de usar este medicamento y póngase inmediatamente en contacto con su médico.
- Infecciones/cirugía: Si usted o el niño desarrollan una nueva infección o están a punto de someterse a una intervención de cirugía mayor, su médico podría tener interés en controlar el tratamiento con este medicamento.
- Infecciones/diabetes: Informe a su médico si usted o el niño tienen historial de infecciones recurrentes o si padecen diabetes u otros trastornos que aumenten el riesgo de infección.
- Infecciones/monitorización: Informe a su médico de cualquier viaje reciente fuera de la región europea. Si usted o el niño desarrollan síntomas de una infección tales como fiebre, escalofríos o tos, notifíqueselo a su médico inmediatamente. Su médico debe decidir si continuar monitorizándole a usted o al niño para ver la presencia de infecciones después de que usted o el niño dejen el tratamiento con Nepexto.
- Tuberculosis: Ya que se han notificado casos de tuberculosis en pacientes tratados con Nepexto, su médico examinará los signos y síntomas de tuberculosis antes de empezar con este medicamento. Esto puede incluir una historia médica minuciosa, radiografía-torácica y una prueba de tuberculosis. La realización de estos análisis debe ser registrada en la Tarjeta para el Paciente. Es muy importante que le diga a su médico si usted o el niño han tenido tuberculosis, o si han estado en contacto directo con alguien que ha tenido tuberculosis. Si los síntomas de tuberculosis (tales como tos persistente, pérdida de peso, apatía, fiebre moderada), o alguna otra infección aparece durante o después del tratamiento, informe a su médico inmediatamente.
- Hepatitis B: Informe a su médico si usted o el niño tienen o han tenido hepatitis B alguna vez. Su médico debe hacerle la prueba de la hepatitis B antes de que usted o el niño comiencen el tratamiento con este medicamento. El tratamiento con Nepexto puede reactivar la hepatitis B en pacientes que hayan estado previamente infectados por el virus de la hepatitis B. Si esto ocurre, debe dejar de usar este medicamento.
- Hepatitis C: Informe a su médico si usted o el niño tienen hepatitis C. Su médico puede querer monitorizar el tratamiento con este medicamento en el caso de que la infección empeore.
- Trastornos de la sangre: Informe inmediatamente a su médico si usted o el niño tienen signos o síntomas tales como, fiebre persistente, dolor de garganta, hematomas, sangrado o palidez. Tales síntomas pueden ser debidos a la existencia de un problema sanguíneo grave que haga necesaria la interrupción del tratamiento con Nepexto.
- Trastornos del sistema nervioso y de la visión: Informe a su médico si usted o el niño presentan esclerosis múltiple, neuritis óptica (inflamación de los nervios ópticos) o mielitis transversa (inflamación de la médula espinal). Su médico decidirá si este medicamento es un tratamiento adecuado.
- Insuficiencia cardiaca congestiva: Informe a su médico si usted o el niño tienen antecedentes de insuficiencia cardiaca congestiva (el músculo del corazón no bombea la sangre como debería), porque este medicamento debe usarse con precaución en esas circunstancias.
- Cáncer: Informe a su médico si usted tiene o ha tenido linfoma (un tipo de cáncer sanguíneo) o cualquier otro cáncer antes de que se le administre este medicamento. Los pacientes con artritis reumatoide grave, que han tenido la enfermedad durante mucho tiempo, pueden correr un riesgo mayor que el promedio de desarrollar linfoma. Los niños y adultos que están tomando este medicamento pueden tener un riesgo incrementado de desarrollar linfoma u otro cáncer. Algunos pacientes adolescentes y niños que han recibido etanercept u otros medicamentos que funcionan de la misma manera que etanercept han desarrollado cánceres, incluyendo tipos inusuales, que algunas veces dieron como resultado la muerte. Algunos pacientes que reciben Nepexto han desarrollado cáncer de piel. Informe a su médico si usted o el niño desarrollan cualquier cambio en el aspecto de la piel o crecimientos en la piel.
- Varicela: Informe a su médico si usted o el niño están expuestos a la varicela mientras utilizan este medicamento. Su médico determinará si es apropiado el tratamiento preventivo para la varicela.
- Alcoholismo: No use este medicamento para el tratamiento de hepatitis relacionada con alcoholismo. Informe a su médico si usted o el niño que está a su cuidado tienen un historial de alcoholismo.
- No se recomienda este medicamento para el tratamiento de granulomatosis de Wegener, una enfermedad inflamatoria rara. Informe a su médico si usted o el niño que está a su cuidado tienen granulomatosis de Wegener.
- Medicamentos antidiabéticos: Informe a su médico si usted o el niño tienen diabetes o están tomando medicamentos para tratar la diabetes. Su médico puede decidir si usted o el niño necesitan menos medicamento antidiabético mientras toman este medicamento.
Niños y adolescentes
- Vacunaciones: Si es posible, los niños deben tener actualizadas todas las vacunaciones antes de utilizar Nepexto. Algunas vacunas, como la vacuna de la polio oral, no se deben administrar mientras se está utilizando este medicamento. Consulte a su médico antes de utilizar usted o el niño cualquier vacuna.
No se debe usar Nepexto en niños y adolescentes que pesen menos de 62,5 kg.
Nepexto no se debe usar en niños menores de 2 años con poliartritis u oligoartritis extendida, en niños menores de 12 años con artritis relacionada con entesitis o artritis psoriásica, ni en niños menores de 6 años con psoriasis.
Uso de Nepexto con otros medicamentos
Informe a su médico o farmacéutico si usted o el niño están utilizando, han utilizado recientemente o podrían tener que utilizar cualquier otro medicamento (incluyendo sulfasalazina), incluso aquellos no prescritos por su médico.
Usted o el niño no deben usarNepexto junto con medicamentos que contengan los principios activos anakinra o abatacept.
Embarazo y lactancia
Nepexto solo debe utilizarse durante el embarazo si es claramente necesario. Consulte a su médico si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada.
Si ha recibido Nepexto durante el embarazo, su bebé puede presentar un mayor riesgo de contraer una infección. Además, en un estudio se observaron más defectos de nacimiento cuando la madre había recibido etanercept durante el embarazo, en comparación con las madres que no habían recibido este medicamento ni otros similares (antagonistas del TNF), pero no hubo ningún patrón en los tipos de defectos de nacimiento notificados. Otro estudio no encontró un mayor riesgo de defectos congénitos cuando la madre había recibido etanercept durante el embarazo. Su médico le ayudará a decidir si los beneficios del tratamiento superan el riesgo potencial para su bebé.
Consulte a su médico si desea dar el pecho mientras está en tratamiento con Nepexto. Es importante que informe al pediatra y a otros profesionales sanitarios sobre el uso de Nepexto durante el embarazo y la lactancia antes de que su bebé reciba cualquier vacuna.
Conducción y uso de máquinas
No se espera que el uso de Nepexto afecte a la capacidad para conducir y usar máquinas.
Nepexto contiene sodio
Este medicamento contiene menos de 1 mmol (23 mg) de sodio por cada dosis, esto es, esencialmente “exento de sodio”.
3. Cómo usar Nepexto
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, pregunte a su médico o farmacéutico.
Si estima que la acción de Nepexto es demasiado fuerte o débil, comuníqueselo a su médico o farmacéutico.
Uso en adultos
Artritis reumatoide, artritis psoriásica y espondiloartritis axial, incluida la espondilitis anquilosante
La dosis habitual es de 25 mg administrados dos veces a la semana o de 50 mg administrados una vez a la semana, en forma de inyección bajo la piel.
Sin embargo, su médico puede determinar una frecuencia alternativa a la que inyectar Nepexto.
Psoriasis en placas
La dosis habitual es de 25 mg dos veces a la semana o 50 mg una vez a la semana.
Alternativamente, pueden administrarse 50 mg dos veces a la semana durante un máximo de 12 semanas, seguido de 25 mg dos veces a la semana o 50 mg una vez a la semana.
Su médico decidirá cuánto tiempo debe usar Nepexto y si necesita una repetición del tratamiento en función de su respuesta. Si Nepexto no tiene efecto sobre su enfermedad después de 12 semanas, su médico puede indicarle que deje de usar este medicamento.
Uso en niños y adolescentes
La dosis adecuada y la frecuencia de dosificación dependerán del peso corporal y de la enfermedad del niño o adolescente.
El médico le indicará como actuar para preparar y medir la dosis adecuada para el niño de etanercept.
No se debe usar Nepexto en niños y adolescentes que pesen menos de 62,5 kg.
Se encuentran disponibles otros medicamentos que contienen etanercept con una formulación adecuada para niños.
Para poliartritis u oligoartritis extendida en pacientes a partir de 2 años de edad, o artritis relacionada con entesitis o artritis psoriásica en pacientes a partir de 12 años, la dosis habitual es 0,4 mg de etanercept por kg de peso corporal (hasta un máximo de 25 mg) dos veces a la semana, o 0,8 mg de etanercept por kg de peso corporal (hasta un máximo de 50 mg) una vez a la semana.
Para psoriasis en pacientes a partir de 6 años de edad, la dosis habitual es 0,8 mg de etanercept por kg de peso corporal (hasta un máximo de 50 mg) una vez a la semana. Si Nepexto no tiene efecto sobre la enfermedad del niño después de 12 semanas, su médico puede indicarle que deje de usar este medicamento.
Forma y vía de administración
Nepexto se administra mediante una inyección bajo la piel (mediante uso subcutáneo).
En la sección 7: “Instrucciones de uso”, se incluyen instrucciones detalladas para la preparación e inyección de Nepexto.
La solución no debe mezclarse con ningún otro medicamento.
Para que le ayude a recordar, puede ser útil anotar en un diario qué días de la semana debe utilizar Nepexto.
Si usa más Nepexto del que debe
Si usted usa más Nepexto del que debiera (bien por inyectar una cantidad elevada en una única ocasión o bien por usarlo con mucha frecuencia), debería hablar con un médico o farmacéutico inmediatamente. Lleve siempre consigo el estuche del medicamento aunque esté vacío.
Si olvidó inyectarse Nepexto
Si se le olvida una dosis, debería inyectarla tan pronto como usted lo recuerde, a no ser que la próxima dosis esté programada para el día siguiente, en cuyo caso deberá omitir la dosis olvidada. A continuación, continúe inyectando el medicamento en el(los) día(s) habitual(es). Si no lo recuerda hasta el día en que debe administrarse la dosis siguiente, no se inyecte una dosis doble (dos dosis en el mismo día) para compensar la dosis olvidada.
Si interrumpe el tratamiento con Nepexto
Sus síntomas pueden volver tras la interrupción del tratamiento.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Reacciones alérgicas
Si observa alguna de las siguientes reacciones, no se inyecte más Nepexto. Informe a su médico inmediatamente o acuda al Servicio de Urgencias del hospital más cercano.
- Dificultad para tragar o respirar.
- Hinchazón de la cara, garganta, manos y pies.
- Sensación de nerviosismo o ansiedad, palpitaciones, enrojecimiento súbito de la piel y/o sensación de calor.
- Erupción grave, picor o urticaria (ronchas prominentes de la piel, enrojecidas o pálidas, acompañadas a menudo de picor).
Las reacciones alérgicas graves son raras. Sin embargo, cualquiera de los síntomas anteriores, puede ser indicio de una reacción alérgica a este medicamento, por lo que usted debe buscar atención sanitaria de urgencia inmediatamente.
Efectos adversos graves
Si usted nota alguno de los efectos siguientes, usted o el niño pueden necesitar atención médica de urgencia.
- Signos de infecciones graves, tales como fiebre alta que puede ir acompañada de tos, falta de aliento, escalofrío, debilidad, o de una zona dolorida, sensible, enrojecida y con sensación de calor en la piel o articulaciones.
- Signos de trastornos sanguíneos, tales como hemorragia, hematomas o palidez.
- Signos de trastornos del sistema nervioso, tales como entumecimiento u hormigueo, alteraciones de la visión, dolor ocular o aparición de debilidad en un brazo o pierna.
- Signos de insuficiencia cardiaca o empeoramiento de la insuficiencia cardiaca, tales como fatiga o falta de aliento con la actividad, hinchazón de los tobillos, sensación de plenitud en el cuello o en el abdomen, falta de aliento durante la noche o tos, color azulado de las uñas o alrededor de los labios.
- Signos del cáncer: el cáncer puede afectar a cualquier parte del cuerpo incluyendo la piel y la sangre, y los posibles signos dependerán del tipo y localización del cáncer. Estos signos pueden ser, entre otros, pérdida de peso, fiebre, hinchazón (con o sin dolor), tos persistente, presencia de bultos o engrosamientos en la piel.
- Signos de reacciones autoinmunes (en las que se desarrollan anticuerpos que pueden dañar tejidos normales del cuerpo) tales como dolor, picor, debilidad y respiración, pensamiento, sensación, o visión anormal.
- Signos de lupus o síndrome tipo lupus tales como cambios de peso, erupción persistente, fiebre, dolor de los músculos o articulaciones o cansancio.
- Signos de inflamación de los vasos sanguíneos tales como dolor, fiebre, enrojecimiento o calor de la piel, o picor.
Estos efectos adversos son raros o poco frecuentes, pero son estados graves (algunos de ellos en raras ocasiones pueden ser mortales). Si ocurre algo de lo mencionado anteriormente, informe a su médico inmediatamente o acuda al Servicio de Urgencias del hospital más cercano.
A continuación se listan los efectos adversos conocidos de etanercept, agrupados por orden decreciente de frecuencia:
- Muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
Infecciones (incluyendo resfriado, sinusitis, bronquitis, infecciones del tracto urinario e infecciones de la piel); reacciones en el lugar de inyección (incluyendo hemorragia, hematoma, enrojecimiento, picor, dolor e hinchazón) (no se producen con tanta frecuencia después del primer mes de tratamiento; algunos pacientes han desarrollado reacción en el sitio de inyección utilizado recientemente); y dolor de cabeza.
- Frecuentes(pueden afectar a hasta 1 de cada 10 personas):
Reacciones alérgicas; fiebre; picores; anticuerpos dirigidos contra los tejidos normales (formación de autoanticuerpos).
- Poco frecuentes(pueden afectar a hasta 1 de cada 100 personas):
Infecciones graves (incluyendo neumonía, infecciones no superficiales de la piel, infecciones de las articulaciones, infección de la sangre e infecciones generalizadas); empeoramiento de la insuficiencia cardiaca congestiva; bajo recuento de glóbulos rojos, bajo recuento de glóbulos blancos, bajo recuento de neutrófilos (un tipo de glóbulos blancos); bajo número de plaquetas; cáncer de piel (excluyendo melanoma); hinchazón localizada de la piel (angioedema); urticaria (ronchas prominentes de la piel, enrojecidas o pálidas, acompañadas a menudo de picor); inflamación ocular, psoriasis (nueva o empeoramiento); inflamación de los vasos sanguíneos afectando a múltiples órganos; aumento de las enzimas hepáticas en los análisis de sangre (en pacientes que también reciben tratamiento con metotrexato, el aumento de las enzimas hepáticas es frecuente);calambre y dolor abdominal, diarrea, pérdida de peso o sangre en heces (signos de problemas intestinales).
- Raros(pueden afectar a hasta 1 de cada 1.000 personas):
Reacciones alérgicas graves (incluyendo hinchazón localizada grave de la piel y respiración jadeante); linfoma (un tipo de cáncer sanguíneo); leucemia (cáncer que afecta a la sangre y médula ósea); melanoma (un tipo de cáncer de piel); de forma combinada bajo recuento de glóbulos rojos, glóbulos blancos y plaquetas; trastornos del sistema nervioso (con debilidad muscular grave y signos y síntomas similares a los de la esclerosis múltiple o inflamación de los nervios ópticos o de la médula espinal); tuberculosis; insuficiencia cardiaca congestiva de nueva aparición; convulsiones; lupus o síndrome tipo lupus (los síntomas pueden incluir erupción persistente, fiebre, dolor de las articulaciones y cansancio); erupción cutánea, que puede conducir a la formación grave de ampollas y a que se pele la piel; reacciones liquenoides (erupción cutánea pruriginosa rojiza-morada y/o líneas gruesas blanco-grisáceas en las mucosas); inflamación del hígado causada por el sistema inmunológico (hepatitis autoinmune; en pacientes que también reciben tratamiento con metotrexato, la frecuencia es poco frecuente); trastorno inmunológico que puede afectar a los pulmones, la piel y los ganglios linfáticos (sarcoidosis); inflamación o cicatrización de los pulmones (en pacientes que también reciben tratamiento con metotrexato, la frecuencia de inflamación o cicatrización de los pulmones es poco frecuente), daño a los pequeños filtros dentro de los riñones, lo que da lugar a una función renal deficiente (glomerulonefritis).
- Muy raros (pueden afectar a hasta 1 de cada 10.000 personas):
Insuficiencia de la médula ósea para producir células sanguíneas cruciales.
- Frecuencia no conocida(no puede estimarse a partir de los datos disponibles):
Carcinoma de células de Merkel (un tipo de cáncer de piel); Sarcoma de Kaposi, un cáncer poco común relacionado con la infección por el virus del herpes humano 8. El sarcoma de Kaposi suele manifestarse con mayor frecuencia como lesiones cutáneas de color púrpura; activación excesiva de glóbulos blancos asociada con la inflamación (síndrome de activación de macrófagos); reactivación de hepatitis B (una infección del hígado); empeoramiento de una enfermedad llamada dermatomiositis (inflamación y debilidad de los músculos acompañada de erupción cutánea).
Otros efectos adversos en niños y adolescentes
Los efectos adversos observados en niños y adolescentes, así como sus frecuencias, son similares a los anteriormente descritos.
Comunicación de efectos adversos
Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: incluido enel Anexo V.
Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Nepexto
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y la pluma precargada después de “EXP”. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 °C y 8 °C). No congelar.
Mantener las plumas precargadas en el embalaje exterior para protegerlas de la luz.
Después de retirar la pluma precargada de la nevera, espere aproximadamente 30 minutos para que la solución de Nepexto en la pluma precargada alcance la temperatura ambiente. No calentar de ninguna otra manera. Úsela de inmediato.
Nepexto se puede conservar fuera de la nevera a una temperatura máxima de 25 °C, y durante un único periodo de hasta cuatro semanas; tras el cual, el medicamento no se puede refrigerar de nuevo. Nepexto se debe desechar si no ha sido usado en las cuatro semanas siguientes a su retirada de la nevera. Es recomendable que anote la fecha en la que Nepexto se ha retirado de la nevera y la fecha a partir de la cual Nepexto se debe desechar (no superior a 4 semanas desde la retirada del envase de la nevera).
Inspeccione la solución en la pluma. Debe ser entre transparente y opalescente, incolora o de color amarillo, y puede contener pequeñas partículas de proteína blancas o casi transparentes. Este es el aspecto normal de Nepexto. No utilice la solución si está descolorida o turbia, o si contiene partículas diferentes a las arriba descritas. Si le preocupa el aspecto de la solución, póngase en contacto con su farmacéutico.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Nepexto
- El principio activo es etanercept. Cada pluma precargada contiene 50 mg de etanercept.
- Los demás componentes son citrato de sodio, fosfato diácido de sodio dihidratado, glicina, sacarosa, cloruro sódico y agua para inyección.
Aspecto del producto y contenido del envase
Nepexto se presenta como una pluma precargada que contiene una solución entre transparente y opalescente, incolora o de color amarillo para inyección.
Nepexto se encuentra disponible en envases que contienen 4 o 12 plumas precargadas, . Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Biosimilar Collaborations Ireland Limited
Unit 35/36
Grange Parade,
Baldoyle Industrial Estate,
Dublin 13
DUBLIN
Irlanda
D13 R20R
El responsable de la fabricación
Biosimilar Collaborations Ireland Limited
Block B, The Crescent Building, Santry Demesne
Dublin
D09 C6X8
Irlanda
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Biocon Biologics Belgium BV Tél/Tel: 0080008250910 | Lietuva Biosimilar Collaborations Ireland Limited Tel: 0080008250910 |
| Luxembourg/Luxemburg Biocon Biologics France S.A.S Tél/Tel: 0080008250910 |
Ceská republika Biocon Biologics Germany GmbH Tel: 0080008250910 | Magyarország Biosimilar Collaborations Ireland Limited Tel.: 0080008250910 |
Danmark Biocon Biologics Finland OY Tlf: 0080008250910 | Malta Biosimilar Collaborations Ireland Limited Tel.: 0080008250910 |
Deutschland Biocon Biologics Germany GmbH Tel: 0080008250910 | Nederland Biocon Biologics France S.A.S Tel: 0080008250910 |
Eesti Biosimilar Collaborations Ireland Limited Tel: 0080008250910 | Norge Biocon Biologics Finland OY Tlf: +47 800 62 671 |
Ελλáδα Biocon Biologics Greece ΜΟΝΟΠΡΟΣΩΠΗ Ι.Κ.Ε Τηλ.: 0080008250910 | Österreich Biocon Biologics Germany GmbH Tel: 0080008250910 |
España Biocon Biologics Spain S.L. Tel: 0080008250910 | Polska Biosimilar Collaborations Ireland Limited Tel: 0080008250910 |
France Biocon Biologics France S.A.S Tel: 0080008250910 | Portugal Biocon Biologics Spain S.L. Tel: 0080008250910 |
Hrvatska Biocon Biologics Germany GmbH Tel: 0080008250910 | România Biosimilar Collaborations Ireland Limited Tel: 0080008250910 |
Ireland Biosimilar Collaborations Ireland Limited Tel: 1800 777 794 | Slovenija Biosimilar Collaborations Ireland Limited Tel: 0080008250910 |
Ísland Biocon Biologics Finland OY Sími: +345 800 4316 | Slovenskárepublika Biocon Biologics Germany GmbH Tel: 0080008250910 |
Italia Biocon Biologics Spain S.L. Tel: 0080008250910 | Suomi/Finland Biocon Biologics Finland OY Puh/Tel: 99980008250910 |
Κúπρος Biosimilar Collaborations Ireland Limited Τηλ: 0080008250910 | Sverige Biocon Biologics Finland OY Tel: 0080008250910 |
Latvija Biosimilar Collaborations Ireland Limited Tel: 0080008250910 |
Fecha de la última revisión de este prospecto:
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos:http://www.ema.europa.eu,
- Instrucciones de uso
Lea las instrucciones de uso antes de comenzar a usar Nepexto y cada vez que recibe una reposición de su receta. Puede contener nueva información.
- No intente autoinyectarse a menos que su médico o enfermero(a) le hayan mostrado cómo hacerlo.
El envase no incluye:
- Toallita impregnada en alcohol
- Gasa y tiritas
- Recipiente para eliminación de objetos punzantes
Partes del dispositivo
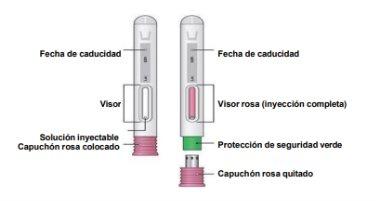
- Preparación para la inyección
Busque una superficie plana, limpia y bien iluminada y reúna todos los elementos necesarios.
Por favor, lea el apartado 5, en el que se incluyen las instrucciones para la conservación de Nepexto. Si tiene alguna duda sobre la conservación, póngase en contacto con su médico, enfermero(a) o farmacéutico para una información más detallada.
| |
Examine el medicamento a través del visor.
| |
Retire una pluma precargada del envase que está guardado en la nevera y déjelo a temperatura ambiente durante al menos 30 minutos antes de inyectarlo. Esto es importante para que el medicamento pueda inyectarse más fácil y de manera más cómoda.
| |
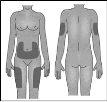
La pluma precargada se utiliza para inyección subcutánea. Debe inyectarse en el muslo, el abdomen o en la parte posterior del bazo (consulte la imagen a la derecha). Alterne el lugar de cada inyección. |
|
Si aplica la inyección en el abdomen, elija un lugar que se encuentre, como mínimo, a 5 cm del ombligo. |
|
- Pasos para la inyección
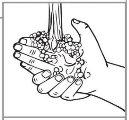
Paso 1: Lávese las manos con agua y jabón. |
|
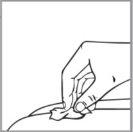
Paso 2: Limpie la piel del lugar de inyección con una toallita empapada en alcohol. Ver la sección 4: “Elegir un lugar de inyección” para obtener información sobre cómo elegir el lugar de inyección.
|
|
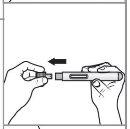
Paso 3: Tire del capuchón de la aguja en línea recta y deséchelo en el recipiente para residuos o en el contenedor para objetos punzantes.
|
|
Paso 4: Estire suavemente la piel en el lugar de inyección limpio. Coloque la pluma precargada aproximadamente a 90 grados de la piel.
|
|
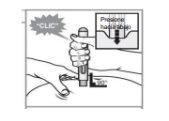
Paso 5: Presione firmemente la pluma precargada en el lugar para comenzar a aplicar la inyección. El dispositivo hará clic cuando comience la inyección. Continúe sosteniendo firmemente la pluma precargada en el lugar. El dispositivo hará clic una segunda vez. |
|
Paso 6: Después del segundo clic, cuente lentamente hasta 15 para asegurarse de completar la inyección.
|
|
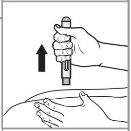
Paso 7: Retire la pluma vacía de la piel. La protección de la aguja cubrirá la aguja por completo. Compruebe que el vástago del émbolo sea rosa en el visor para asegurarse de que se haya administrado la dosis completa. |
|
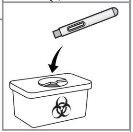
Eliminación: Elimine la pluma vacía en un contenedor para objetos punzantes aprobado. Pida al proveedor de asistencia sanitaria instrucciones sobre cómo eliminar correctamente el contenedor de objetos punzantes lleno. Los contenedores para la eliminación de objetos punzantes pueden adquirirse en una farmacia.
|
|
- Cuidado de la zona de inyección
Si se produce sangrado en el lugar de inyección, presione la zona con una gasa.
- No friccione el lugar de inyección.
Si es necesario, cubra el lugar de la inyección con una tirita.
Si tiene alguna duda o necesita más información, póngase en contacto con su médico, enfermero(a) o farmacéutico.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a NEPEXTO 50 MG SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE, 25 mgPrincipio activo: EtanerceptFabricante: Samsung Bioepis Nl B.V.Requiere recetaForma farmacéutica: INYECTABLE, 50 MGPrincipio activo: EtanerceptFabricante: Samsung Bioepis Nl B.V.Requiere recetaForma farmacéutica: INYECTABLE, 50MGPrincipio activo: EtanerceptFabricante: Samsung Bioepis Nl B.V.Requiere receta
Médicos online para NEPEXTO 50 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de NEPEXTO 50 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes