KETISAL 0,25 MG/ML COLIRIO EN SOLUCION


Cómo usar KETISAL 0,25 MG/ML COLIRIO EN SOLUCION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Ketisal 0,25 mg/ml colirio en solución
Ketotifeno
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Ketisal 0,25 mg/ml colirio en solución y para qué se utiliza
- Qué necesita saber antes de empezar a usar Ketisal 0,25 mg/ml colirio en solución
- Cómo usar Ketisal 0,25 mg/ml colirio en solución
- Posibles efectos adversos
- Conservación de Ketisal 0,25 mg/ml colirio en solución
- Contenido del envase e información adicional
1. Qué es Ketisal y para qué se utiliza
Este medicamento contiene el principio activo ketotifeno, que es una molécula antialérgica.
Este medicamento se usa para tratar los síntomas oculares de la fiebre del heno.
2. Qué necesita saber antes de empezar a usar Ketisal
No use Ketisal
- Si es alérgico (hipersensible) al ketotifeno o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a usar este medicamento.
Niños
No se recomienda el uso de Ketisal en niños menores de 3 años de edad.
Otros medicamentos y Ketisal
Si necesita aplicarse en los ojos cualquier otro medicamento junto con este medicamento, espere como mínimo 5 minutos entre la aplicación de cada producto.
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento, incluidos los medicamentos obtenidos sin receta. Esto es especialmente importante en el caso de medicamentos que se usan para tratar:
- depresión, ansiedad y trastornos del sueño
- alergia (por ejemplo, antihistamínicos)
Ketisal con alimentos, bebida y alcohol
El uso de este medicamento puede aumentar los efectos del alcohol.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Este medicamento se puede usar durante la lactancia.
Conducción y uso de máquinas
Este medicamento puede causar visión borrosa o adormecimiento. Si esto sucede, espere hasta que estos efectos hayan desaparecido antes de conducir o usar máquinas.
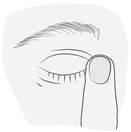
3. Cómo usar Ketisal
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada en adultos, pacientes de edad avanzada y niños (a partir de 3 años de edad), es de una gota en el ojo (u ojos) afectado(s) dos veces al día (por la mañana y por la noche).
Instrucciones de uso
1a
|
|
|
|
|
|
5
|
|
1-5 |
|
Si tiene cualquier otra duda sobre el uso de este medicamento, consulte a su médico o farmacéutico.
Si usa más Ketisal del que debe
No hay peligro si ha usado más de una gota en el ojo o si accidentalmente ha ingerido este medicamento. En caso de duda, consulte a su médico o farmacéutico.
En caso de sobredosis o ingestión accidental, consulte a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida
Si olvidó usar Ketisal
Si olvidó usar este medicamento debe aplicarse el tratamiento tan pronto como se acuerde, y a la dosis recomendada (una gota por ojo, dos veces al día). No use una dosis doble para compensar las dosis olvidadas.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Se han comunicado los siguientes efectos adversos.
Frecuentes (pueden afectar hasta 1 de cada 10 pacientes)
- irritación ocular o dolor en el ojo
- inflamación en el ojo
Poco frecuentes (pueden afectar hasta 1 de cada 100 pacientes)
- visión borrosa cuando se aplican las gotas en el ojo
- sequedad en el ojo
- alteración en el párpado
- conjuntivitis
- aumento de la sensibilidad de los ojos a la luz
- hemorragia visible en la zona blanca del ojo
- dolor de cabeza
- somnolencia
- erupción (que puede también producir picor)
- eczema (picor, enrojecimiento, erupción con escozor)
- sequedad de boca
- reacción alérgica (incluyendo hinchazón de la cara y los párpados) e incremento de la gravedad de una afección alérgica ya existente como asma y eczema.
Si experimenta efectos adversos graves, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano, https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Ketisal
Mantener este medicamento fuera de la vista y del alcance de los niños.
No conservar a temperatura superior a 25°C.
Una vez abierto el frasco, se puede guardar durante 3 meses.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y el frasco después de CAD. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Ketisal
- El principio activo es ketotifeno (como hidrógeno fumarato).
Cada ml de solución contiene 0,345 mg de hidrógeno fumarato de ketotifeno, lo que corresponde a 0,25 mg de ketotifeno.
- Los demás componentes (excipientes) son hialuronato de sodio, glicerol (E 422), hidróxido de sodio (E 524) y agua purificada.
Aspecto del producto y contenido del envase
Este medicamento es una solución transparente, incolora o ligeramente amarillenta. La solución está envasada en un frasco de plástico blanco de 10 ml cerrado con un gotero. Cada frasco de plástico contiene 10 ml de solución.
Titular de la autorización de comercialización
HORUS PHARMA
22 Allé Camille Muffat
INEDI 5
06200 Nice
Francia
Responsable de la fabricación
PHARMASTER
Zone Industrielle de Krafft Erstein
FRANCIA
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Alemania: Ketazed 0,25 mg/ml, Augentropfen, Lösung
Bélgica: Ketazed 0,25 mg/ml collyre en solution / Ketazed 0,25 mg/ml oogdruppels, oplossing / Ketazed 0,25 mg/ml Augentropfen, Lösung
Dinamarca: Ketazed, øjendråber, opløsning
España: Ketisal 0,25 mg/ml colirio en solución
Finlandia: Ketazed 0,25 mg/ml silmätipat, liuos
Francia: Ketazed 0,25 mg/mL, collyre en solution
Holanda : Ketazed 0,25 mg/ml oogdruppels, oplossing
Italia: KETAZED 0,25 mg/ml, collirio, soluzione
Luxembourgo: : Ketazed 0,25 mg/ml collyre en solution
Noruega: Ketazed 0,25 mg/ml øyedråper, oppløsning
Rumania: Ketazed 0,25 mg/ml picaturi oftalmice, solu?ie
Suecia: Ketazed 0,25 mg/ml ögondroppar, lösning
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Horus Pharma Ibérica, S.L.U.
Gran Vía Carlos III, 98, 6º
08028 Barcelona - España
Fecha de la última revisión de este prospecto: Junio 2025
La información detallada de este medicamento está disponible en la página web de la Agencia Española del Medicamento y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia7.02 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a KETISAL 0,25 MG/ML COLIRIO EN SOLUCIONForma farmacéutica: COLIRIO, 0,25 mg/mlPrincipio activo: ketotifenFabricante: Laboratoires TheaRequiere recetaForma farmacéutica: COLIRIO, 0,25mg/mlPrincipio activo: ketotifenFabricante: Pharma Stulln GmbhRequiere recetaForma farmacéutica: COLIRIO, 0,25 mg/mlPrincipio activo: ketotifenFabricante: Nutra Essential Otc S.L.Requiere receta
Médicos online para KETISAL 0,25 MG/ML COLIRIO EN SOLUCION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de KETISAL 0,25 MG/ML COLIRIO EN SOLUCION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes




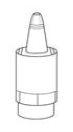
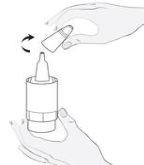 1b
1b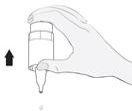 2
2 3
3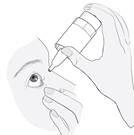 4
4