
HYDROXOCOBALAMIN BASE 1 mg/ml INJECTABLE SOLUTION

How to use HYDROXOCOBALAMIN BASE 1 mg/ml INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Hydroxocobalamin Basi 1 mg/ml Solution for Injection
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Hydroxocobalamin Basi and what is it used for
- What you need to know before you use Hydroxocobalamin Basi
- How to use Hydroxocobalamin Basi
- Possible side effects
- Storage of Hydroxocobalamin Basi
- Contents of the Pack and Further Information
1. What is Hydroxocobalamin Basi and what is it used for
Hydroxocobalamin Basi is a form of Vitamin B12, an essential vitamin necessary for the production of red blood cells.
Hydroxocobalamin Basi is used to prevent and treat certain types of anemia, including Addison's pernicious anemia and other types of anemia resulting from a vitamin B12 deficiency.
2. What you need to know before you use Hydroxocobalamin Basi
Do not use Hydroxocobalamin Basi
- if you are allergic to hydroxocobalamin or any of the other ingredients of this medicine (listed in section 6).
If any of the above applies to you, talk to your doctor, pharmacist, or nurse.
Warnings and Precautions
Talk to your doctor, pharmacist, or nurse before you start using Hydroxocobalamin Basi.
Talk to your doctor if:
- you are pregnant, plan to become pregnant, or are breast-feeding;
- you have megaloblastic anemia, a blood disorder characterized by the presence of abnormally large red blood cells.
Other Medicines and Hydroxocobalamin Basi
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
In particular, tell your doctor if you are taking any of the following medicines:
- Antibiotics (for infection treatment) or antimetabolites (medicines that stop cell division, such as mercaptopurine for leukemia), as these treatments may interfere with tests to measure vitamin B12 levels in blood or urine;
- An antibiotic called chloramphenicol, as it may reduce the effect of Hydroxocobalamin Basi;
- Oral contraceptives.
Pregnancy and Breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Hydroxocobalamin Basi should not be used during pregnancy to treat a type of anemia called megaloblastic anemia, unless you also have a vitamin B12 deficiency.
Hydroxocobalamin Basi passes into breast milk, but it is unlikely to harm your baby.
Driving and Using Machines
It is unlikely that this medicine will affect your ability to drive or use machines. However, some people may feel dizzy or drowsy when receiving treatment with hydroxocobalamin. If this happens, do not drive or use machines.
Hydroxocobalamin Basi contains Sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially "sodium-free".
3. How to use Hydroxocobalamin Basi
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. If you are unsure, talk to your doctor or pharmacist again.
You will be given Hydroxocobalamin Basi by injection into a muscle. Your doctor will choose the right dose for you. This medicine may be given once or may be repeated every two days, weekly, or monthly, depending on how much your body needs.
The recommended doses in severe cases, by deep injection into a muscle, are as follows:
Adults and Children
- Treatment of anemia:
- Without neurological symptoms (nervous system):
Initially 250 – 1,000 micrograms, every two days for 1 to 2 weeks, then 250 micrograms weekly until blood tests are normal.
Maintenance dose: 1,000 micrograms (1 ampoule) every 2 to 3 months.
- With neurological symptoms:
1,000 micrograms (1 ampoule) every two days until improvement is seen.
Maintenance dose: 1,000 micrograms (1 ampoule) every 2 months.
- Prevention of anemia:
1,000 micrograms (1 ampoule) every 2 to 3 months.
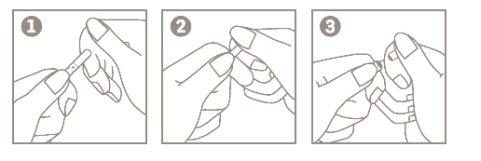
Instructions for Opening the Ampoule:
- Hold the body of the ampoule between your thumb and index finger, with the point facing upwards;
- Place the index finger of your other hand holding the top part of the ampoule. Place your thumb over the point;
- With your index fingers close together, press the area of the point to open the ampoule.
Medical Exams
While you are being treated with this medicine, your doctor will want you to have blood tests done periodically. This is to make sure the medicine is working correctly and that the dose you are receiving is right for you.
If you use more Hydroxocobalamin Basi than you should
If you think you have used too much Hydroxocobalamin Basi, it is unlikely that you will need special treatment. However, if you feel unwell or experience side effects, you should talk to your doctor.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
In case of overdose or accidental ingestion, talk to your doctor or pharmacist immediately or call the Toxicological Information Service, phone 91 562 04 20, stating the medicine and the amount ingested.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop using Hydroxocobalamin Basi and tell your doctor immediately if you experience any of the following symptoms:
- Difficulty breathing;
- Swelling of the eyelids, face, or lips;
- Rash or itching, especially if it covers the whole body.
Other Side Effects
Frequency not known: cannot be estimated from the available data
- Low potassium levels in the blood and irregular heartbeat during the initial stages of treatment;
- Thrombocytosis (high platelet count in the blood);
- Rash;
- Blisters;
- Pain at the injection site;
- Hardening of the skin at the injection site;
- Necrosis (skin destruction) around the injection site;
- Hives;
- Feeling unwell or sick;
- Being sick;
- Diarrhea;
- Urinating pink or red-colored urine;
- Dizziness;
- Headache;
- Tremor;
- Fever;
- Chills;
- Flushing;
- Pain.
Reporting of Side Effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Hydroxocobalamin Basi
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the packaging and on the ampoule label after EXP. The expiry date is the last day of the month stated.
Do not store above 25°C.
The product should not be used if it is not a clear red solution or if it contains visible particles.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the Pack and Further Information
Composition of Hydroxocobalamin Basi
- The active substance is hydroxocobalamin. Each 1 ml ampoule contains hydroxocobalamin chloride equivalent to 1,000 micrograms of hydroxocobalamin.
- The other ingredients (excipients) are sodium chloride, glacial acetic acid, and water for injections.
Appearance and Pack Size of the Product
Hydroxocobalamin Basi is a clear red solution, free from visible particles, presented in glass ampoules. Pack size: 5 and 10 ampoules of 1 ml.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Laboratórios Basi - Indústria Farmacêutica, S.A.
Parque Industrial Manuel Lourenço Ferreira, Lote 15
3450-232 Mortágua, Portugal
Tel.: +351 231 920 250
Fax: +351 231 921 055
Email: [email protected]
Manufacturer
Laboratórios Basi - Indústria Farmacêutica, S.A.
Parque Industrial Manuel Lourenço Ferreira Lotes 8, 15 e 16
3450-232 Mortágua
Portugal
Local Representative
Laphysan SAU,
Calle Anabel Segura 11,
Complejo Empresarial Albatros, Edificio A, Planta 4, puerta D
28108 Alcobendas (Madrid)
This medicine is authorized in the Member States of the European Economic Area under the following names:
Portugal: Hidroxocobalamina Basi
Ireland: Hydroxocobalamin Basi 1000 microgram/ml solution for injection
Norway: Hydroxocobalamin Basi
Spain: Hidroxocobalamina Basi 1 mg/ml solution for injection
Date of the last revision of this leaflet: January 2023
Other Sources of Information
Detailed and up-to-date information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to HYDROXOCOBALAMIN BASE 1 mg/ml INJECTABLE SOLUTIONDosage form: INJECTABLE, 10 mg hydroxocobalamin/2 mlActive substance: hydroxocobalaminManufacturer: Aristo Pharma Iberia S.L.Prescription requiredDosage form: TABLET, 1000 microgramsActive substance: cyanocobalaminManufacturer: Italfarmaco S.A.Prescription requiredDosage form: TABLET, 0.4 mg folic acid; 0.002 mg cyanocobalaminActive substance: cyanocobalamin, combinationsManufacturer: Itf Abacus Farma S.A.Prescription required
Online doctors for HYDROXOCOBALAMIN BASE 1 mg/ml INJECTABLE SOLUTION
Discuss questions about HYDROXOCOBALAMIN BASE 1 mg/ml INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















