
HIDROFEROL 0.266 mg ORAL SOLUTION

How to use HIDROFEROL 0.266 mg ORAL SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What Hidroferol 0.266 mg Oral Solution is and what it is used for
- What you need to know before taking Hidroferol 0.266 mg Oral Solution
- How to take Hidroferol 0.266 mg Oral Solution
- Possible side effects
- Storage of Hidroferol 0.266 mg Oral Solution
- Contents of the pack and further information
Introduction
Package Leaflet: Information for the User
Hidroferol 0.266 mg Oral Solution
calcifediol monohydrate
Read the entire package leaflet carefully before starting to take this medication, as it contains important information for you.
This medication is subject to medical prescription and should only be taken under medical supervision.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor, pharmacist, or nurse.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What Hidroferol 0.266 mg Oral Solution is and what it is used for
- What you need to know before taking Hidroferol 0.266 mg Oral Solution
- How to take Hidroferol 0.266 mg Oral Solution
- Possible side effects
- Storage of Hidroferol 0.266 mg Oral Solution
- Contents of the pack and further information
1. What Hidroferol 0.266 mg Oral Solution is and what it is used for
It contains a form of vitamin D, calcifediol, used to treat vitamin D deficiency and related problems. Vitamin D acts in the human body, among other actions, by increasing calcium absorption.
Hidroferol 0.266 mg Oral Solution is indicated for the treatment of vitamin D deficiency in adults and to prevent vitamin D deficiency in adults with identified risks, such as patients with malabsorption syndrome, chronic kidney disease - mineral bone disease (CKD-MBD), or other identified risks.
It is also used to treat bone loss (osteoporosis), in combination with other medications, in patients with vitamin D deficiency or at risk of vitamin D deficiency.
2. What you need to know before taking Hidroferol 0.266 mg Oral Solution
Do not take Hidroferol 0.266 mg Oral Solution:
- if you are allergic to calcifediol or any other component of this medication (listed in section 6).
- if you have hypercalcemia (high blood calcium levels) or hypercalciuria (high urine calcium levels).
- if you have kidney stone formation (calcium stones).
- if you have hypervitaminosis D (excess vitamin D in the body).
Warnings and precautions
Consult your doctor or pharmacist before starting to take Hidroferol 0.266 mg Oral Solution.
- Do not exceed the recommended daily amounts of vitamin D supplements, such as this medication, as this may cause intoxication (see section 3, "If you take more Hidroferol 0.266 mg Oral Solution than you should").
- While taking this medication or before starting, your doctor may instruct you to have blood or urine tests to monitor your calcium, phosphorus, and other parameter levels.
- Patients with kidney disease require special caution and should be closely monitored by their doctor, with periodic tests.
- Patients with heart disease require special caution and should be frequently supervised by their doctor to monitor blood calcium levels, especially those being treated with cardiac glycosides (see section "Other medications and Hidroferol 0.266 mg Oral Solution").
- If you have hypoparathyroidism (insufficient parathyroid hormone function), the action of this medication may be reduced.
- If you have kidney stone formation, your doctor should monitor your blood calcium levels.
- Patients with prolonged immobilization may require lower doses of this medication.
- Patients with sarcoidosis (a disease with nodules, usually on the skin), tuberculosis, or other diseases with nodules should be cautious with this medication, as they are at higher risk of adverse effects at lower-than-recommended doses. Periodic tests should be performed to monitor blood and urine calcium levels.
- Interference with laboratory tests: if you are going to have any diagnostic tests (including blood tests, urine tests, skin tests using allergens, etc.), inform your doctor that you are taking this medication, as it may alter the results. For example, in some cholesterol tests.
Children and adolescents
The safety and efficacy of calcifediol in children and adolescents under 18 years of age have not been established. No data are available.
For use in children, the medication Hidroferol 0.1 mg/ml oral solution is available on the market, with a lower dose concentration.
Other medications and Hidroferol 0.266 mg Oral Solution
Inform your doctor or pharmacist if you are using, have recently used, or may need to use any other medication, including those purchased without a prescription.
Some medications may affect how this medication works. On the other hand, its active ingredient, calcifediol monohydrate, may affect the efficacy of other medications taken at the same time.
Therefore, interactions may occur with the following medications:
- Medications used to treat epilepsy (such as phenytoin, phenobarbital, and primidone) and other enzyme-inducing medications (which may decrease the effect of Hidroferol).
- Medications for the heart and/or hypertension, such as cardiac glycosides, thiazide diuretics, or verapamil.
- Colestyramine, colestipol (for cholesterol), orlistat (for obesity): the intake of these medications and calcifediol should be separated by at least 2 hours.
- Mineral oil or paraffin (laxatives): it is recommended to use another type of laxative or separate the intake of both medications.
- Some antibiotics (such as penicillin, neomycin, and chloramphenicol).
- Magnesium salts.
- Other products containing Vitamin D.
- Calcium supplements.
- Corticosteroids (anti-inflammatory medications).
Taking Hidroferol 0.266 mg Oral Solution with food and beverages
Some foods and beverages are supplemented with vitamin D. This should be taken into account, as the effects of these foods may add up to the effects of this medication and become excessive.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Do not take this medication during pregnancy.
Do not take this medication while breastfeeding your child.
Driving and using machines
This medication does not affect the ability to drive vehicles or operate machines.
3. How to take Hidroferol 0.266 mg Oral Solution
The Hidroferol 0.266 mg Oral Solution ampoule is EXCLUSIVELY for oral administration.
Hidroferol 0.266 mg Oral Solution should NOT be administered intramuscularly and/or intravenously.
Follow the administration instructions for this medication exactly as indicated by your doctor. If in doubt, consult your doctor or pharmacist again.
DO NOT take Hidroferol 0.266 mg Oral Solution DAILY.Do not take more medication or take it more frequently than indicated by your doctor (weekly, biweekly, or monthly). If you do, you may increase the risk of an overdose.
The recommended doses are:
- Treatment of vitamin D deficiency and prevention of vitamin D deficiency in patients with identified risks: one drinkable ampoule (0.266 mg of calcifediol monohydrate), once a month.
- As a complement to specific medication for bone loss: one drinkable ampoule (0.266 mg of calcifediol monohydrate), once a month.
There are high-risk populations for vitamin D deficiency where higher doses may be necessary. After analyzing the degree of deficiency, your doctor may consider a dose of one ampoule every two weeks or every week.
Your doctor should periodically monitor your calcium and vitamin D levels, usually before starting treatment and 3-4 months after initiating treatment. According to the indication, doses will be reduced or spaced out over time when symptoms improve or vitamin D deficiency is corrected.
Oral administration.
The contents of the ampoule can be taken alone or diluted in a little water, milk, or juice.
The instructions for opening and using the ampoules are as follows:
Shake the ampoule before use.
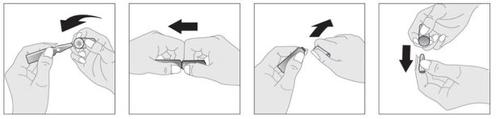
Insert the head of the glass ampoule into the opening facilitator cylinder. Hold the body of the ampoule between your thumb and index finger with the white dot facing up.
Place the index finger of your other hand under the cylinder that covers the head of the ampoule, and your thumb above it, as indicated in the figure, so that the index fingers of both hands touch each other under the ampoule.
Keep the hand holding the ampoule still and press the head down with your thumb to open the ampoule with a sharp movement.
Once the ampoule is open, remove the head of the ampoule from the cylinder for later reuse.
If you think the action of this medication is too strong or too weak, inform your doctor or pharmacist.
If you take more Hidroferol 0.266 mg Oral Solution than you should
If you take more of this medication than the dose indicated by your doctor (overdose) and/or for a prolonged period, you may experience hypercalcemia (high blood calcium levels) and hyperphosphatemia (high phosphate levels in blood and urine), leading to possible kidney failure. Some symptoms of toxicity may appear soon, while others may appear after some time.
Initial symptoms include: weakness, fatigue, headache, loss of appetite, dry mouth, digestive disorders such as vomiting, abdominal cramps, constipation, or diarrhea, increased thirst; increased urination, muscle pain.
Symptoms that appear after some time may include: itching, weight loss, growth retardation in children, kidney disorders, intolerance to sunlight, conjunctivitis, increased cholesterol, transaminases, pancreatitis, calcification (calcium salt deposits) in blood vessels and other tissues such as tendons and muscles, increased blood pressure, mental disorders, irregular heartbeats.
Symptoms of overdose usually improve or disappear when treatment is discontinued, but if the intoxication is severe, it could cause kidney or heart failure.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or go to a medical center, or call the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount ingested (or bring the medication with you).
If you forget to take Hidroferol 0.266 mg Oral Solution
Do not take a double dose to make up for forgotten doses.
Take the forgotten dose as soon as possible; then return to your regular dosing schedule.
If you have any other questions about the use of this medication, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
Side effects may occur if excessive doses are taken or with a frequency higher than indicated by the doctor, which may cause hypercalcemia (high blood calcium levels) and hypercalciuria (high urine calcium levels); see section 3 for a description of symptoms.
Other side effects include allergic reactions such as itching, local swelling, difficulty breathing, and skin redness.
Reporting side effects
If you experience any type of side effect, consult your doctor, pharmacist, or nurse, even if it is a possible side effect not listed in this package leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use: www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Hidroferol 0.266 mg Oral Solution
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date stated on the packaging after CAD. The expiration date is the last day of the month indicated.
No special storage conditions are required.
Medications should not be disposed of through wastewater or household waste. Deposit the packaging and medications you no longer need at the SIGRE collection point in your pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Contents of the pack and further information
Composition of Hidroferol 0.266 mg Oral Solution
- The active ingredient is calcifediol monohydrate. Each ampoule contains 0.266 mg of calcifediol monohydrate.
- The other ingredients (excipients) are: medium-chain triglycerides and alpha-tocopherol acetate.
Appearance of the product and pack contents
Hidroferol 0.266 mg is an oral solution; it is a clear, colorless or slightly yellowish, viscous liquid, free from visible impurities; it is presented in glass ampoules.
Each pack contains 10 drinkable ampoules.
Other presentations of Hidroferol:
Hidroferol 0.1 mg/ml oral solution drops.
Hidroferol 0.266 mg soft capsules.
Hidroferol Shock 3 mg oral solution.
Marketing authorization holder
Faes Farma, S.A.
Autonomia Etorbidea, 10
48940 Leioa (Bizkaia)
Spain
Manufacturer
Faes Farma Portugal, S.A.
Rua Elias Garcia, 28
Amadora-P-2700
Portugal
Date of the last revision of this package leaflet: November 2023
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price10.32 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to HIDROFEROL 0.266 mg ORAL SOLUTIONDosage form: CAPSULE, 0.266 mgActive substance: calcifediolManufacturer: Laboratorios Normon S.A.Prescription requiredDosage form: CAPSULE, 0.266 mgActive substance: calcifediolManufacturer: Laboratorios Normon S.A.Prescription requiredDosage form: ORAL SOLUTION/SUSPENSION DROPS, 0.1 mgActive substance: calcifediolManufacturer: Faes Farma S.A.Prescription required
Online doctors for HIDROFEROL 0.266 mg ORAL SOLUTION
Discuss questions about HIDROFEROL 0.266 mg ORAL SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















