
ГОНАСІ КІТ 5000 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ


Інструкція із застосування ГОНАСІ КІТ 5000 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ
Вступ
Опис: інформація для пацієнта
Гоназі Кіт 5 000 МОпорошок і розчинник для ін'єкційної розчини
гонадотропін людини хоріонічний
?Цей лікарський засіб підлягає додатковому моніторингу, що прискорить виявлення нової інформації про його безпеку. Ви можете допомогти, повідомляючи про будь-які побічні ефекти, які ви можете мати. Остання частина розділу 4 містить інформацію про те, як повідомляти про ці побічні ефекти.
Прочитайте уважно весь опис перед початком використання цього лікарського засобу, оскільки він містить важливу інформацію для вас.
- Збережіть цей опис, оскільки вам може знадобитися знову його прочитати.
- Якщо у вас є якісь питання, проконсультуйтеся з вашим лікарем або фармацевтом.
- Цей лікарський засіб призначений тільки вам, і не слід давати його іншим людям, навіть якщо вони мають相同ні симптоми, оскільки це може їм нашкодити.
- Якщо ви відчуваєте побічні ефекти, проконсультуйтеся з вашим лікарем або фармацевтом, навіть якщо це можливі побічні ефекти, які не перелічені в цьому описі. Див. розділ 4.
- У цьому описі Гоназі Кіт 5 000 МО порошок і розчинник для ін'єкційної розчини називається Гоназі Кіт.
Зміст опису
- Що таке Гоназі Кіт і для чого він використовується
- Що потрібно знати перед початком використання Гоназі Кіту
- Як використовувати Гоназі Кіт
- Можливі побічні ефекти
- Зберігання Гоназі Кіту
- Зміст упаковки та додаткова інформація
1. Що таке Гоназі Кіт і для чого він використовується
Що такеГоназі Кіт
Цей лікарський засіб містить високоочищений гонадотропін людини хоріонічний, гормон, отриманий з людської сечі, який належить до сім'ї "гонадотропінів", тобто гормонів, пов'язаних з репродукцією та фертильністю.
Для чого використовуєтьсяГоназі Кіт:
Цей лікарський засіб використовується:
- Для допомоги у розвитку та дозріванні кількох фолікулів (кожен з яких містить яйцеклітину) у жінок, які проходять процедури допоміжної репродукції (процедура, яка може допомогти вам завагітіти), такі як "екстракорпоральне запліднення" (ЕЗ)
- Для допомоги у вивільненні яйцеклітини з яєчника (індукція овуляції) у жінок, які не можуть продукувати яйцеклітини ("анівуляція") або продукують дуже мало ("оліговуляція").
Цей лікарський засіб повинен використовуватися під наглядом вашого лікаря, якщо тільки ваш лікар не дав вам інших інструкцій.
2. Що потрібно знати перед початком використання Гоназі Кіту
Не використовуйтеГоназі Кіт:
- якщо ви алергічні на гонадотропін людини хоріонічний або на будь-який інший компонент цього лікарського засобу (перелічені в розділі 6)
- якщо у вас є захворювання, які не лікувалися, що впливають на щитоподібну, пітую або наднирникову залози
- якщо у вас є пухлина яєчника, матки або молочної залози
- якщо у вас є обставини, які роблять неможливим нормальну вагітність, наприклад, недостатність яєчників, відсутність матки, передчасна менопауза, обструкція фаллопієвих труб, міоми матки або інші аномалії репродуктивних органів
- якщо ви нещодавно мали вагінальне кровотеча невідомої причини.
Проконсультуйтеся з вашим лікарем або фармацевтом, якщо будь-яка з цих умов стосується вас, оскільки цей лікарський засіб може не бути підходящим для вас.
Попередження та застереження
Ваш лікар повинен перевірити, перед початком лікування, чи ваші репродуктивні органи нормальні.
Проконсультуйтеся з вашим лікарем перед використанням цього лікарського засобу, якщо ви маєте або мали будь-які з наступних станів:
- аномалії репродуктивних органів
- хронічні захворювання (наприклад, цукровий діабет, захворювання серцево-судинної системи тощо).
- ускладнення судин (тобто підвищений ризик утворення кров'яних згустків, сімейна історія або попередні кров'яні згустки, надмірна вага).
Медичні дослідження
Під час періоду до десяти днів після введення цього лікарського засобу тест на вагітність може показати хибний позитивний результат.
Під час лікування цим лікарським засобом може виникнути наступне:
Синдром гіперстимуляції яєчників (СГЯ)
Лікування гонадотропними гормонами, такими як цей лікарський засіб, може викликати синдром гіперстимуляції яєчників (СГЯ). Це серйозна стан, при якій яєчники надмірно стимулюються і фолікули, які ростуть, стають більші, ніж зазвичай. У рідких випадках важкий СГЯ може бути потенційно смертельним. Тому дуже важливо проводити тісний нагляд вашого лікаря. Для перевірки ефекту лікування ваш лікар може призначити ультразвукове дослідження яєчників. Ваш лікар також може перевірити рівні гормонів у крові. (Див. також розділ 4, "Можливі побічні ефекти").
СГЯ викликає раптове накопичення рідини в черевній (живіт) та грудній порожнині, що може викликати утворення кров'яних згустків. Повідоміть вашому лікарю негайно, якщо у вас є:
- сильна припухлість і біль у черевній області (живіт)
- нудота (нудота)
- воміта
- раптове збільшення ваги через накопичення рідини
- діарея
- зменшення об'єму сечі
- утруднення дихання.
Торсія яєчника
Торсія яєчника - це скручування яєчника. Скручування яєчника може викликати припинення кровотоку до яєчника.
Перед початком лікування цим лікарським засобом важливо повідомити вашому лікарю, якщо:
- ви раніше мали СГЯ
- ви вагітні або думаєте, що можете бути вагітною
- ви мали операцію на черевній порожнині (черевну хірургію)
- ви мали торсію яєчника
- у вас є кісти або ви мали кісти на яєчнику чи яєчниках.
Кров'яні згустки (тромбоз)
Вагітність збільшує ймовірність утворення кров'яного згустку.
Якщо у вас є фактори ризику утворення кров'яного згустку (наприклад, надмірна вага, або якщо у вас є сімейна історія або попередні кров'яні згустки), ймовірність утворення згустку в судині може збільшитися під час лікування ЕЗ.
Кров'яні згустки можуть викликати серйозні захворювання, такі як:
- закупорка легенів (легенева емболія)
- інсульт
- серцевий напад
- зменшення кровотоку до життєво важливих органів, що може викликати пошкодження органів
- зменшення кровотоку (глибока венозна тромбоз) в вашій руці або нозі, що може викликати втрату руки або ноги.
Багатоплідна вагітність, вади розвитку, викидень або ускладнення вагітності
Якщо лікування цим лікарським засобом призведе до вагітності, існує більша ймовірність мати двійнята або багатоплідну вагітність. Багатоплідна вагітність збільшує ризик для здоров'я як матері, так і дітей, до та після народження. У жінок, які проходять лікування фертильності, існує трохи більший ризик викидня або вагітності поза маткою (ектопічна вагітність). Тому ваш лікар повинен провести ультразвукове дослідження на початку, щоб виключити можливість вагітності поза маткою. Багатоплідна вагітність більш ймовірна, якщо ви приймаєте інші лікарські засоби, які сприяють овуляції (наприклад, хМГ).
Не відомо, чи лікування ЕЗ викликає вади розвитку чи деякі захворювання статевих органів.
Діти та підлітки
Цей лікарський засіб не повинен використовуватися у дітей чи підлітків.
Інші лікарські засоби та Гоназі Кіт
Повідоміть вашому лікарю або фармацевту, якщо ви приймаєте або нещодавно приймали будь-які інші лікарські засоби, навіть без рецепта. Це особливо важливо, якщо ви приймаєте лікарські засоби, які:
- сприяють овуляції (наприклад, хМГ);
- містять кортизон, особливо у високих дозах.
Вагітність та годування грудьми
Не використовуйте цей лікарський засіб, якщо ви вагітні або годуєте грудьми. Якщо ви думаєте, що можете бути вагітною, проконсультуйтеся з вашим лікарем перед використанням цього лікарського засобу.
Водіння транспортних засобів та використання машин
Цей лікарський засіб не впливає на вашу здатність водити транспортні засоби чи використовувати машини.
Гоназі Кіт містить натрій
Цей лікарський засіб містить менше 1 ммоль натрію (23 мг) на реконституїровану розчин; тобто, він практично "не містить натрію".
3. Як використовувати Гоназі Кіт
Гоназі Кіт - це порошок, який повинен бути розчинений у рідині (розчиннику) перед використанням; він вводиться ін'єкцією під шкіру (підшкірно) або в м'яз (внутрішньом'язово). Розчин отримується шляхом поєднання розчинника з порошком і повинен використовуватися негайно після його приготування.
Слідуйте точно інструкціям щодо введення лікарського засобу, вказаним вашим лікарем. У разі сумнівів проконсультуйтеся з вашим лікарем або фармацевтом.
Як вводити Гоназі Кіт:
Цей лікарський засіб вводиться ін'єкцією під вашу шкіру (підшкірно) або в ваш м'яз (внутрішньом'язово).
Кожна ампула повинна використовуватися лише один раз, і ін'єкція повинна використовуватися негайно після її приготування.
Рекомендована доза цього лікарського засобу становить 5 000 МО або 10 000 МО для введення через 24-48 годин після досягнення оптимальної стимуляції фолікулярного розвитку.
Після відповідної консультації та навчання ваш лікар може попросити вас вводити лікарський засіб самостійно.
У разі першого введення ваш лікар повинен:
- пояснити, як підготувати відповідну дозу для ін'єкції
- показати, як підготувати ін'єкційну розчин
- показати зони, в які можна вводити ін'єкцію
- дозволити вам практикувати введення підшкірної ін'єкції самостійно.
Перед тим, як вводити лікарський засіб самостійно, прочитайте наступні інструкції уважно.
Як підготувати Гоназі Кіт за допомогою 1 ампули порошку
Розчин повинен підготуватися негайно перед ін'єкцією. Кожна ампула призначена лише для одного використання.
Цей лікарський засіб повинен бути реконституїрованний лише з розчинником, який поставляється в упаковці, згідно з наступними інструкціями:
Пункт 1
Вимийте руки перед підготуванням розчину. Використайте чисту поверхню для підготування. важливо, щоб ваші руки та матеріали, які ви використовуєте, були якнайчистішими.
Пункт 2
- Помістіть наступні матеріали на чисту поверхню:
- два вати, змочені спиртом (не входять до складу упаковки)
- ампула, яка містить порошок Гоназі Кіту
- розчинник у попередньо заповненому шприці
- довга голка для реконституївання та для внутрішньом'язової ін'єкції.
- коротка голка для підшкірної ін'єкції.
Пункт 3
- Видаліть тільки кришечку шприца;
- Вставте голку для реконституївання (довгу голку) з її захисним ковпачком у шприц і перевірте, чи голка добре встановлена, щоб уникнути втрати розчину. У разі втрати розчину спробуйте закріпити голку легким поворотом.
- Помістіть шприц на чисту поверхню.
- Уникайте дотику до голки.
Пункт 4
- Видаліть пластикову кришечку з ампули Гоназі Кіту, натиснувши її легенько вгору.
- Витріть гумовий ковпачок ватою, змоченою спиртом, і чекайте, поки він висохне.
Пункт 5
- Візьміть шприц, видаліть захисний ковпачок з голки і вставте голку крізь центр гумового ковпачка ампули Гоназі Кіту.
- Натисніть поршень вниз з силою і повільно введіть розчинник у ампулу з порошком крізь гумовий ковпачок, щоб виливати всю розчин у ампулу, яка містить порошок.
- НЕ ВИТРЯСАЙТЕ, прокатайте ампулу між пальцями, поки порошок не розчиниться повністю, обережно уникайте утворення піни.
Пункт 6
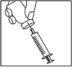
Після розчинення порошку (що, зазвичай, відбувається негайно), повільно завантажте розчин у шприц, як описано нижче:
- З голкою, ще введеною, помістіть ампулу догори дном.
- Переконайтеся, що кінчик голки знаходиться нижче рівня рідини.
- Тягніть за поршень повільно, щоб завантажити всю розчин у шприц.
- Перевірте, чи реконституїрована розчин прозора та безбарвна.
Підготовка більших доз, використання більше 1 ампули порошку
Якщо ваш лікар призначив вам вищу дозу 10 000 МО, її можна отримати, використовуючи дві ампули порошку з попередньо заповненим шприцем розчинника.
Під час підготування 2 ампул лікарського засобу на кінці попереднього пункту 3 завантажте вміст реконституїрованої ампули у шприц і повільно введіть його в другу ампулу. Повторіть пункти 4-6 з іншою ампулою.
Розчин повинен бути прозорим та безбарвним.
Введення вашого лікарського засобу внутрішньом'язово
Для внутрішньом'язових ін'єкцій ваш лікар або медсестра підготує та введе цей лікарський засіб у вашу стегно або сідницю.
Введення вашого лікарського засобу підшкірно
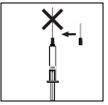 Якщо шприц містить правильну дозу для вас, помістіть захисний ковпачок на голку. Видаліть довгу голку з шприца і замініть її короткою голкою для підшкірної ін'єкції з її захисним ковпачком. Будьте уважні, щоб голка була добре встановлена, і натисніть коротку голку сильно у циліндр шприца, потім поверніть її легенько, щоб забезпечити щільне з'єднання, щоб уникнути втрати розчину.
Якщо шприц містить правильну дозу для вас, помістіть захисний ковпачок на голку. Видаліть довгу голку з шприца і замініть її короткою голкою для підшкірної ін'єкції з її захисним ковпачком. Будьте уважні, щоб голка була добре встановлена, і натисніть коротку голку сильно у циліндр шприца, потім поверніть її легенько, щоб забезпечити щільне з'єднання, щоб уникнути втрати розчину.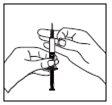 Видаліть захисний ковпачок з голки. Візьміть шприц з голкою вгору і легенько стукніть по бічній стороні шприца, щоб примусити повітряні бульки піднятися вгору;
Видаліть захисний ковпачок з голки. Візьміть шприц з голкою вгору і легенько стукніть по бічній стороні шприца, щоб примусити повітряні бульки піднятися вгору;- Натисніть поршень, поки не з'явиться крапля рідини на кінчику голки.
- НЕ ВИКОРИСТОВУЙТЕ розчин, якщо він містить частинки або є мутним.
Місце ін'єкції:
 Ваш лікар або медсестра вже порадили вам, в якій ділянці вашого тіла потрібно вводити лікарський засіб. Звичайними місцями є стегно або нижня частина черевної порожнини (ділянка живота), нижче пупка.
Ваш лікар або медсестра вже порадили вам, в якій ділянці вашого тіла потрібно вводити лікарський засіб. Звичайними місцями є стегно або нижня частина черевної порожнини (ділянка живота), нижче пупка.- Витріть місце ін'єкції ватою, змоченою спиртом.
- Зігніть і стисніть шкіру сильно. Іншою рукою введіть голку рухом, як при викиданні дротика, під кутом 45° або 90°.
Введення розчину:
- Введіть під шкіру, як вказав ваш лікар.
- Не вводьте прямо в вену.
- Натисніть поршень повільно та постійно, щоб правильно вввести розчин і не пошкодити шкіру.
Витратьте весь час, який вам потрібно, щоб вввести призначений об'єм розчину.
Видалення голки:
- Видаліть шприц швидко і натисніть на місце ін'єкції
- Легкий масаж у ділянці - підтримуючи натиск - допомагає розсіяти розчин лікарського засобу та полегшити будь-який дискомфорт.
Видаліть весь використаний матеріал:
Після закінчення ін'єкції всі голки та порожні шприци повинні бути видалені в відповідний контейнер. Видалення невикористаної розчину та всіх матеріалів, які були в контакті з нею, здійснюється згідно з місцевими правилами.
Якщо ви використали більше Гоназі Кіту, ніж потрібно:
Не відомі ефекти передозування цього лікарського засобу, однак можна очікувати, що виникне синдром гіперстимуляції яєчників (див. "Можливі побічні ефекти"). Якщо ви використали більшу кількість лікарського засобу, ніж потрібно, проконсультуйтеся з вашим лікарем або фармацевтом.
У разі передозування або випадкового прийому проконсультуйтеся негайно з вашим лікарем або фармацевтом або зверніться до служби токсикологічної інформації, телефон: 91 562 04 20, вказавши лікарський засіб та кількість, прийняту.
Якщо ви забули використати Гоназі Кіт:
Якщо ви забули використати лікарський засіб, проконсультуйтеся негайно з вашим лікарем.
Якщо ви перервали лікування Гоназі Кітом:
Проконсультуйтеся з вашим лікарем, якщо ви розглядаєте можливість не використовувати цей лікарський засіб.
Якщо у вас є будь-які інші питання щодо використання цього лікарського засобу, проконсультуйтеся з вашим лікарем або фармацевтом.
4. Можливі побічні ефекти
Як і всі лікарські засоби, цей лікарський засіб може викликати побічні ефекти, хоча не всі люди їх відчувають.
Якщо ви помітите будь-який з наступних серйозних побічних ефектів, припиніть використання Gonasi Kit і негайно зверніться до лікаря, оскільки можливо буде потрібне термінове медичне лікування:
- легка або помірна гіперстимуляція яєчників (синдром гіперстимуляції яєчників), яка проявляється збільшенням розміру яєчників, оваріальними кістами, болем у животі з блювотою та нудотою (див. також розділ 2 у "Синдром гіперстимуляції яєчників"). Це частий побічний ефект (може впливати до 1 з 10 осіб).
- тяжка гіперстимуляція яєчників (синдром гіперстимуляції яєчників) характеризується болем у нижній частині живота (тазу), нудотою, блювотою, збільшенням ваги, накопиченням рідини у животі (асцит) або в грудній порожнині (плевральний випот). Цей епізод рідкісний (може впливати до 1 з 100 осіб).
- переривання оваріального кісти (як рідкісна наслідування важкої гіперстимуляції яєчників, може впливати до 1 з 1 000 осіб)
- утворення тромбів у кровоносних судинах (тромбоемболічні епізоди), як ускладнення синдрому гіперстимуляції яєчників. Цей епізод рідкісний (може впливати до 1 з 1 000 осіб)
- загальні серйозні алергічні реакції, які можуть включати: набухання обличчя, очей, губ, горла або мови, труднощі з диханням, свистіння, висипання на шкірі - рідкісний побічний ефект (може впливати до 1 з 1 000 осіб)
Інші побічні ефекти
Часті побічні ефекти:можуть впливати до 1 з 10 осіб
- Реакція на місці ін'єкції, яка може включати червоніння, синці, набухання, свербіж або біль на місці ін'єкції
- набухання (едем)
- зміни настрою
- головний біль
- біль у грудній порожнині
Рідкісні побічні ефекти:можуть впливати до 1 з 100 осіб
- безсоння (агітація), втома
Рідкісні побічні ефекти:можуть впливати до 1 з 1 000 осіб
- набухання глибоких шарів шкіри, яке часто спостерігається з уртикарією (кропив'янкою)
- загальні висипання на шкірі
Якщо ви відчуваєте будь-який з рідкісних побічних ефектів, зазначених вище, негайно зверніться до свого лікаря, який вирішить, чи потрібно припинити лікування цим лікарським засобом або негайно звернутися до найближчої лікарні.
Звіт про побічні ефекти
Якщо ви відчуваєте будь-який тип побічного ефекту, зверніться до свого лікаря або фармацевта, навіть якщо це можливо побічні ефекти, які не зазначені в цьому описі. Ви також можете повідомити про них безпосередньо через Іспанську систему фармакологічного нагляду за лікарськими засобами для людини: https://www.notificaram.es. Надсилаючи повідомлення про побічні ефекти, ви можете допомогти надати більше інформації про безпеку цього лікарського засобу.
5. Зберігання Gonasi Kit
Тримайте цей лікарський засіб поза зоною видимості та доступу дітей.
Не зберігайте при температурі вище 25°C. Зберігайте флакон та шприц з розчинником у зовнішній упаковці для захисту їх від світла.
Використовуйте негайно після підготовки розчину.
Не використовуйте цей лікарський засіб після закінчення терміну придатності, зазначеного на зовнішній упаковці, флаконі та шприці з розчинником. Якщо термін придатності вказано у вигляді місяць/рік, термін придатності закінчується в останній день зазначеного місяця.
Не використовуйте цей лікарський засіб, якщо ви помітите, що розчин не має прозорого вигляду (туманний або з видимими частинками). Розчин повинен бути прозорим та безбарвним після його реконституції.
Лікарські засоби не повинні викидати у каналізацію чи сміття. Відкладайте упаковку та лікарські засоби, які вам не потрібні, у пункті збору SIGRE  аптеки. У разі сумніву запитайте у свого фармацевта, як позбутися упаковки та лікарських засобів, які вам більше не потрібні. Таким чином, ви допоможете захистити навколишнє середовище.
аптеки. У разі сумніву запитайте у свого фармацевта, як позбутися упаковки та лікарських засобів, які вам більше не потрібні. Таким чином, ви допоможете захистити навколишнє середовище.
6. Зміст упаковки та додаткова інформація
Склад Gonasi Kit
Активний інгредієнт: гонадотропін людини.
Допоміжні речовини у:
- флаконі з порошком: лактоза моногідрат
- шприці з розчинником (хлорид натрію 0,9%): вода для ін'єкційних препаратів, хлорид натрію.
Кожен флакон містить: 5 000 ОД гонадотропіну людини, отриманого з людської сечі.
Вигляд продукту та вміст упаковки
Gonasi Kit представлений у вигляді:
Порошок у флаконі: лioфілізований порошок білого або майже білого кольору
Розчинник у шприці (хлорид натрію 0,9%): прозорий та безбарвний розчин
Одна упаковка містить:
Пластикову підкладку, яка включає порошок у флаконі (скло типу I), закритому кришкою з гумовим затвором та утримуваним на місці кришкою, що легко відкривається.
1 мл розчинника у шприці (скло типу I), 1 довгу голку для реконституції та для інтримускульної ін'єкції та 1 коротку голку для підшкірної ін'єкції.
Багаторазова упаковка, яка містить 10 пластикових підкладок, як описано вище.
Можливо, що будуть реалізовані лише деякі розміри упаковок.
Власник дозволу на торгівлю:
IBSA Farmaceutici Italia srl
Віа Мартірі ді Кефалонія 2
26900 Лоді (Італія)
Відповідальний за виробництво:
IBSA Farmaceutici Italia srl
Віа Мартірі ді Кефалонія, 2
26900 Лоді – Італія
Ви можете запитати додаткову інформацію про цей лікарський засіб, звернувшись до місцевого представника власника дозволу на торгівлю:
Інститут Біохімічний Іберико IBSA S.L.
Авенюда Діагональ 605,
Плант 8, Локал 1,
08028 Барселона (Іспанія)
Цей лікарський засіб дозволений у країнах-членах Європейського економічного простору під наступними назвами: (Доза та лікарська форма ідентичні у всіх країнах, змінюється лише комерційна назва)
Данія: Gonasi Set
Чеська Республіка: Zivafert
Іспанія: Gonasi Kit
Фінляндія: Gonasi Set
Франція: GONADOTROPHINE CHORIONIQUE IBSA
Угорщина: Zivafert Kit
Нідерланди: Gonasi
Норвегія: Gonasi Set
Польща: Zivafert
Швеція: Gonasi Set
Словацька Республіка: Gonasi Kit
Дата останнього перегляду цього опису:Січень 2025
Детальна та оновлена інформація про цей лікарський засіб доступна на сайті Іспанського агентства лікарських засобів та медичних продуктів (AEMPS) http://www.aemps.gob.es.
- Країна реєстрації
- Діючі речовини
- Потрібен рецептТак
- Виробник
- Інформація є довідковою і не є медичною порадою. Перед прийомом будь-яких препаратів обов'язково проконсультуйтеся з лікарем. Oladoctor не несе відповідальності за медичні рішення, прийняті на основі цього контенту.
- Альтернативи до ГОНАСІ КІТ 5000 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУФорма випуску: РОЗЧИН ДЛЯ ІН'ЄКЦІЙ, 150 МО/ 0,25 мл (11 мікрограм/ 0,25 мл)Діючі речовини: follitropin alfaВиробник: Gedeon Richter Plc.Потрібен рецептФорма випуску: РОЗЧИН ДЛЯ ІН'ЄКЦІЙ, 150 МО/ 0,25 мл (11 мікрограм/ 0,25 мл)Діючі речовини: follitropin alfaВиробник: Gedeon Richter Plc.Потрібен рецептФорма випуску: РОЗЧИН ДЛЯ ІН'ЄКЦІЙ, 150 МО/0,25 мл (11 мікрограм/0,25 мл)Діючі речовини: follitropin alfaВиробник: Gedeon Richter Plc.Потрібен рецепт
Аналоги ГОНАСІ КІТ 5000 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ в інших країнах
Найкращі аналоги з тією самою діючою речовиною та терапевтичним ефектом.
Аналог ГОНАСІ КІТ 5000 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ у Польща
Аналог ГОНАСІ КІТ 5000 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ у Україна
Лікарі онлайн щодо ГОНАСІ КІТ 5000 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ
Консультація щодо дозування, побічних ефектів, взаємодій, протипоказань та поновлення рецепта на ГОНАСІ КІТ 5000 МО ПОРОШОК І РОЗЧИННИК ДЛЯ ПРИГОТУВАННЯ ІН'ЄКЦІЙНОГО РОЗЧИНУ – за рішенням лікаря та згідно з місцевими правилами.





