EVRYSDI 0,75 mg/ml POLVO PARA SOLUCION ORAL


Cómo usar EVRYSDI 0,75 mg/ml POLVO PARA SOLUCION ORAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Evrysdi 0,75mg/ml polvo para solución oral
risdiplam
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted o a su hijo, y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si usted o su hijo experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Evrysdi y para qué se utiliza
- Qué necesita saber antes de que usted o su hijo empiece a tomar Evrysdi
- Cómo tomar Evrysdi
- Posibles efectos adversos
- Conservación de Evrysdi
- Contenido del envase e información adicional
1. Qué es Evrysdi y para qué se utiliza
Qué es Evrysdi
Evrysdi es un medicamento que contiene el principio activo risdiplam.
Para qué se utiliza Evrysdi
Evrysdi se utiliza para tratar la atrofia muscular espinal (AME), una enfermedad genética.
Qué es la atrofia muscular espinal
La AME está causada por una cantidad insuficiente en el organismo de una proteína llamada proteína de supervivencia de la neurona motora (SMN). La carencia de proteína SMN puede causarle a usted o a su hijo la pérdida de las neuronas motoras, las cuales son células nerviosas que controlan los músculos. Esto provoca debilidad muscular y pérdida de masa muscular que puede afectar a los movimientos diarios como el control de la cabeza y del cuello, sentarse, gatear y caminar. Los músculos que se utilizan para respirar y tragar también pueden debilitarse.
Cómo funciona Evrysdi
Risdiplam, el principio activo que contiene Evrysdi, actúa ayudando al cuerpo a producir más proteína SMN. Esto se traduce en la pérdida de menos neuronas motoras, lo que puede mejorar el funcionamiento de los músculos de las personas con AME.
En los bebés con AME Tipo 1 tratados en los ensayos clínicos durante 1 año, Evrysdi ha ayudado a:
- aumentar el tiempo de vida y reducir la necesidad de un ventilador para ayudarles a respirar, en comparación con los bebés con AME no tratados (se esperaría que solo el 25% de los bebés no tratados estén vivos sin necesidad de ventilación permanente después de los 14 meses de edad, en comparación con el 85% de los pacientes después de 1 año de tratamiento con Evrysdi),
- mantener la capacidad para alimentarles por la boca en el 83% de los pacientes.
En niños (de pequeños a adolescentes) y adultos con AME Tipo 2 y 3, Evrysdi puede mantener o mejorar el control de los músculos.
2. Qué necesita saber antes de que usted o su hijo empiece a tomar Evrysdi
No tome Evrysdi:
- Si usted o su hijo es alérgico a risdiplam o a alguno de los demás excipientes de este medicamento (incluidos en la sección 6).
En caso de duda, consulte a su médico o farmacéutico antes de que usted o su hijo empiece a tomar Evrysdi.
Advertencias y precauciones
Consulte a su médico, enfermero o farmacéutico antes de que usted o su hijo empiece a tomar Evrysdi.
El tratamiento con Evrysdi puede dañar al bebé en gestación o afectar a la fertilidad masculina. Consulte “Embarazo, anticoncepción, lactancia y fertilidad masculina” para obtener más información.
Otros medicamentos y Evrysdi
Informe a su médico o farmacéutico si usted o su hijo está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento en el futuro.
En particular, informe a su médico, farmacéutico o enfermero si está tomando o ha recibido en el pasado alguno de los siguientes medicamentos:
- metformina – un medicamento que se utiliza para tratar la diabetes tipo II
- medicamentos para el tratamiento de la AME
Embarazo, anticoncepción, lactancia y fertilidad masculina
Embarazo
- No tome Evrysdi si está embarazada. Esto se debe a que tomar este medicamento durante el embarazo podría dañar al bebé en gestación.
- Antes de iniciar el tratamiento con Evrysdi, su médico puede hacerle una prueba de embarazo. Esto se debe a que Evrysdi puede dañar al bebé en gestación.
- Si se queda embarazada durante el tratamiento con Evrysdi, informe a su médico inmediatamente.
Usted y su médico decidirán qué es lo mejor para usted y para el bebé en gestación.
Anticoncepción
Para las mujeres
No se quede embarazada:
- durante el tratamiento con Evrysdi y
- durante un mes después de dejar de tomar Evrysdi.
Hable con su médico sobre los métodos anticonceptivos fiables que se deben usar durante el tratamiento y durante un mes después de dejar de tomar el tratamiento.
Para los hombres
Si su pareja es una mujer en edad fértil, debe evitar el embarazo. Use métodos anticonceptivos fiables (p. ej. preservativos):
- durante el tratamiento con Evrysdi y
- durante 4 meses después de dejar de tomar Evrysdi.
Consulte con su profesional sanitario sobre los métodos anticonceptivos fiables que se deben usar.
Lactancia
No amamante a su hijo mientras esté tomando este medicamento. Esto se debe a que Evrysdi puede pasar a la leche materna y, por tanto, puede dañar al bebé.
Hable con su médico sobre si debe dejar la lactancia o debe dejar de tomar Evrysdi.
Fertilidad masculina
Según hallazgos en animales, Evrysdi puede reducir la fertilidad masculina durante el tratamiento y durante 4 meses después de la última dosis. Si está planeando tener un hijo, consulte a su médico para que le aconseje.
No done esperma durante el tratamiento y durante 4 meses después de la última dosis de Evrysdi.
Conducción y uso de máquinas
Es poco probable que Evrysdi afecte a su capacidad para conducir y utilizar máquinas.
Evrysdi contiene sodio
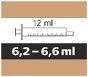
Evrysdi contiene una pequeña cantidad de sodio (sal) - hay menos de 1 mmol de sodio (23 mg) incluso a la dosis diaria máxima de 5 mg (6,6 ml de solución oral de 0,75 mg/ml). Esto significa que es, esencialmente, “exento de sodio” y puede ser utilizado por personas con una dieta baja en sodio.
Evrysdi contiene 0,375 mg de benzoato sódico por ml. El benzoato sódico puede aumentar el riesgo de ictericia (coloración amarillenta de la piel y los ojos) en los recién nacidos (hasta de 4 semanas de edad).
Evrysdi contiene isomaltosa
Evrysdi contiene 2,97 mg de isomaltosa por ml. Si su médico le ha indicado que usted o su hijo padece una intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento.
3. Cómo tomar Evrysdi
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico. Debe recibir Evrysdi en forma de líquido, dentro de un frasco. Si el medicamento del frasco es un polvo, no lo use y póngase en contacto con su farmacéutico.
También debe leer atentamente y seguir el folletoadjunto “Instrucciones de uso” sobre cómo tomar o administrar Evrysdi.
Cuánto Evrysdi tomar
- Adolescentes y adultos:La dosis diaria de Evrysdi es de 5 mg (6,6 ml de la solución oral).
- Bebés y niños:Su médico decidirá la dosis correcta de Evrysdi en función de la edad y el peso de su hijo.
Usted o su hijo debe tomar la dosis diaria que le indique su médico.No modifique la dosis sin hablar con su médico.
Cuándo y cómo tomar Evrysdi
- Evrysdi es un líquido que prepara el farmacéutico y al que se hace referencia en este prospecto como una “solución” o “medicamento”.
- Tome Evrysdi una vez al día aproximadamente a la misma hora todos los día. Esto le ayudará a recordar cuándo tomar el medicamento.
- Beba agua después de tomar el medicamento. No mezcle este medicamento con leche o leche en polvo.
- Tome o administre Evrysdi inmediatamente después de que haya extraido el medicamento en la jeringa oral. Si no se toma en los siguientes 5 minutos, deseche el medicamento de la jeringa oral y extraiga una nueva dosis.
- Si Evrysdi entra en contacto con su piel o la de su hijo, lave la zona con agua y jabón.
Lea el folleto de “Instrucciones de uso”
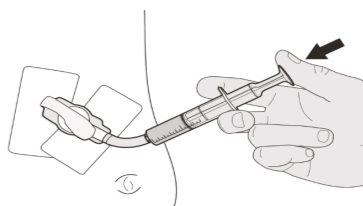
Se incluye un folletode “Instrucciones de uso” en el envase, en el que se indica como extraer la dosis usando la jeringa oral reutilizable que se le ha suministrado. Usted (o su hijo) puede tomar el medicamento:
- por la boca,
- a través de una sonda de gastrostomía o
- a través de una sonda nasogástrica.
Cuánto tiempo debe tomar Evrysdi
El médico le dirá cuánto tiempo debe tomar Evrysdi usted o su hijo. No interrumpa el tratamiento con Evrysdi a menos que el médico se lo indique.
Si usted o su hijo toma más Evrysdi del que debe
Si toma más Evrysdi del que debe, consulte a un médico o acuda al hospital inmediatamente. Lleve consigo el envase del medicamento y este prospecto.
Si usted o su hijo olvida tomar Evrysdi, o vomita después de la administración de la dosis
- Si aún no han transcurrido 6 horas desde el momento en que usted o su hijo toma Evrysdi normalmente, tome la dosis omitida tan pronto como se acuerde.
- Si han transcurrido más de 6 horas desde el momento en que usted o su hijo toma Evrysdi normalmente, sáltese la dosis omitida y tome la siguiente dosis a la hora habitual. No tome una dosis doble para compensar la dosis olvidada.
- Si usted o su hijo vomita después de tomar una dosis de Evrysdi, no tome una dosis adicional. En su lugar, tome la siguiente dosis al día siguiente en su horario habitual.
Si usted derrama Evrysdi
Si usted derrama Evrysdi, seque la zona con una toalla de papel seca y límpiela con jabón y agua. Tire la toalla de papel en la basura y límpiese bien las manos con jabón y agua.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Muy frecuentes:pueden afectar a más de 1 de cada 10 personas
- diarrea
- sarpullido
- dolor de cabeza
- fiebre
Frecuentes: pueden afectar hasta a 1 de cada 10 personas
- nauseas
- úlceras en la boca
- infección de vejiga
- dolor en las articulaciones
El siguiente efecto adverso se ha notificado tras el inicio de la comercialización de Evrysdi, sin embargo, se desconoce la frecuencia con la que ocurre:
- Inflamación de los vasos sanguíneos pequeños que afecta principalmente a la piel (vasculitis cutánea).
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V.* Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Evrysdi
- Mantener este medicamento fuera de la vista y del alcance de los niños.
- Conservar la solución oral en nevera (entre 2 ºC y 8 ºC). Si fuera necesario, usted o su cuidador pueden mantener la solución oral a temperatura ambiente (por debajo de 40 ºC) durante un máximo de 120 horas (5 días) en total. Guarde la solución oral de nuevo en la nevera, cuando ya no sea necesario mantenerla a temperatura ambiente.
- Monitorice el tiempo total que está fuera de la nevera (por debajo de 40 ºC). Como se indica anteriormente, la suma de los intervalos de tiempo fuera de la nevera no debe superar las 120 horas.
- La solución oral es estable durante 64 días después de su preparación por el farmacéutico, siempre que se conserve en nevera entre 2 ºC y 8 ºC. El farmacéutico anotará la fecha de caducidad en la etiqueta del frasco y en el embalaje original en “Desechar después de”. No utilice esta solución después de la fecha indicada en “Desechar después de” o deseche el medicamento si el frasco se ha mantenido a temperatura ambiente (por debajo de 40 ºC) durante más de 120 horas (5 días) en total.
- Deseche el medicamento si en cualquier momento el frasco se ha conservado por encima de 40 ºC.
- Conservar el medicamento en el frasco original para protegerlo de la luz.
- Conservar el frasco de medicamento en vertical, con el tapón perfectamente cerrado.
- Una vez extraído el medicamento en la jeringa oral, use Evrysdi de manera inmediata. No conserve en la jeringa la solución de Evrysdi.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Evrysdi
- El principio activo de la solución oral es risdiplam.
- Cada ml de la solución oral contiene 0,75 mg de risdiplam.
- Los demás excipientes son manitol (E 421), isomaltosa (E 953), aroma de fresa, ácido tartárico (E 334), benzoato sódico (E 211), macrogol/polietilenglicol 6000, sucralosa, ácido ascórbico (E 300) y edetato disódico dihidrato (ver Sección 2 “Evrysdi contiene sodio” y “Evrysdi contiene isomaltosa”).
Aspecto de Evrysdiy contenido del envase
- Polvo para solución oral, el cual se proporciona como solución oral después de su preparación por el farmacéutico.
- La solución oral es de color amarillo verdoso a amarillo y con sabor a fresa, el volumen de la solución es de 80 ml.
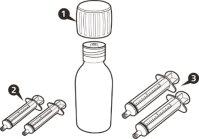
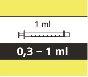
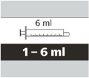
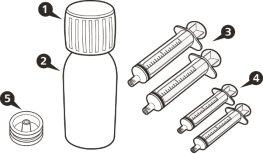
- Cada envase contiene 1 frasco, 1 adaptador para el frasco y jeringas orales reutilizables de color ámbar (2 de 1 ml, 2 de 6 ml y una de 12 ml), con graduación para ayudar a extraer la dosis correcta.
Titular de la autorización de comercialización
Roche Registration GmbH
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Alemania
Responsable de la fabricación
Roche Registration AG
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
|
Ceskárepublika Roche s. r. o. Tel: +420 - 2 20382111 | Magyarország Roche (Magyarország) Kft. Tel: +36 1 279 4500 |
Danmark Roche Pharmaceuticals A/S Tlf: +45 - 36 39 99 99 | Malta (see Ireland ) |
Deutschland RochePharmaAG Tel: +49 (0) 7624 140 | Nederland Roche Nederland B.V. Tel: +31 (0) 348 438050 |
Eesti Roche Eesti OÜ Tel: + 372 - 6 177 380 | Norge Roche Norge AS Tlf: +47 - 22 78 90 00 |
Ελλ?δα Roche (Hellas) A.E. Τηλ: +30 210 61 66 100 | Österreich Roche Austria GmbH Tel: +43 (0) 1 27739 |
España Roche Farma S.A. Tel: +34 - 91 324 81 00 | Polska Roche Polska Sp.z o.o. Tel: +48 - 22 345 18 88 |
France Roche Tél: +33 (0) 1 47 61 40 00 | Portugal Roche Farmacêutica Química, Lda Tel: +351 - 21 425 70 00 |
Hrvatska Roche d.o.o. Tel: +385 1 4722 333 | România Roche România S.R.L. Tel: +40 21 206 47 01 |
Ireland Roche Products (Ireland) Ltd. Tel: +353 (0) 1 469 0700 | Slovenija Roche farmacevtska družba d.o.o. Tel: +386 - 1 360 26 00 |
Ísland Roche Pharmaceuticals A/S c/o Icepharma hf Sími: +354 540 8000 | Slovenská republika Roche Slovensko, s.r.o. Tel: +421 - 2 52638201 |
Italia Roche S.p.A. Tel: +39 - 039 2471 | Suomi/Finland Roche Oy Puh/Tel: +358 (0) 10 554 500 |
K?προς Γ.Α.Σταμ?της & Σια Λτδ. Τηλ: +357 - 22 76 62 76 | Sverige Roche AB Tel: +46 (0) 8 726 1200 |
Latvija Roche Latvija SIA Tel: +371 - 6 7039831 | United Kingdom (Northern Ireland) Roche Products (Ireland) Ltd. Tel: +44 (0) 1707 366000 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu.
Instrucciones de uso - Administración
Evrysdi 0,75mg/ml polvo para solución oral
risdiplam
Asegúrese de leer y comprender estas Instrucciones de usoantes de empezar a usar Evrysdi. Estas instrucciones muestran cómo preparar y administrar Evrysdi mediante una jeringa oral, sonda de gastrostomía (sonda G) o sonda nasogástrica (sonda NG).
En caso de duda sobre cómo utilizar Evrysdi, contacte con su médico o farmacéutico.
Evrysdi debería venir como líquido en un frasco cuando lo reciba. El farmacéutico prepara la solución oral de Evrysdi. Si el medicamento del frasco es un polvo, nolo utilice y contacte con su farmacéutico.
Información importante sobre Evrysdi ? Pida a su médico o farmacéutico que le enseñe qué jeringa oral utilizar y cómo medir la dosis diaria prescrita. ? Utilice siempre las jeringas orales reutilizables suministradas en el envase para medir la dosis diaria. ? Contacte con su médico o farmacéutico si la(s) jeringa(s) oral(es) se pierde/pierden o daña/dañan. Ellos le indicarán cómo continuar tomando el medicamento. ? Consulte “Cómo seleccionar la jeringa oral correcta para la dosis de Evrysdi”. Pregunte a su farmacéutico si tiene dudas sobre cómo seleccionar la jeringa oral correcta. ? Si el adaptador para el frasco no se encuentra en el frasco, noutilice Evrysdi y contacte con su farmacéutico.
? No useEvrysdi después de la fecha de Desechar después deque figura en la etiqueta del frasco o en el caso de que usted o su cuidador haya mantenido el frasco a temperatura ambiente (por debajo de 40 ºC) durante más de 120 horas (5 días) en total. Pregunte a su farmacéutico por la fecha de Desechar después desi no está escrita en la etiqueta del frasco.
? Nomezcle Evrysdi con leche o leche en polvo. ? Nouse Evrysdi si el frasco o la jeringa oral están dañados. ? Eviteel contacto de Evrysdi con la piel. Si Evrysdi entra en contacto con la piel, lave la zona con agua y jabón. ? Si derrama Evrysdi, seque la zona con una toalla de papel seca y limpia y, a continuación, lávela con agua y jabón. Tire la toalla de papel a la basura y lávese bien las manos con agua y jabón. ? Si no queda suficiente Evrysdi en el frasco para la dosis prescrita, deseche el frasco con el resto de Evrysdi y la jeringa oral usada conforme a los requisitos locales; use un nuevo frasco de Evrysdi para obtener una dosis completa. No mezcleEvrysdi del frasco nuevo con el frasco que está utilizando actualmente. | ||||||||
Cada caja de EVRYSDI contiene (ver figuraA):
|
Figura A | |||||||
Conservación de Evrysdi Consulte la sección 5 “Conservación de Evrysdi” del prospecto para obtener la información completa. | ||||||||
Cómo seleccionar la jeringa oral correcta para la dosis de Evrysdi
| ||||||||
Cómo extraer la dosis de Evrysdi | ||||||||
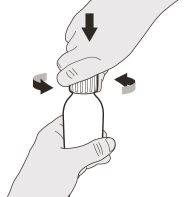
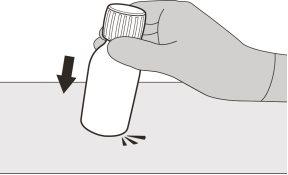
Figura B | Paso A1 Retire el tapón presionando hacia abajo y, a continuación, haga girar el tapón a la izquierda (en sentido contrario a las agujas del reloj) (ver figura B). No deseche el tapón. | |||||||
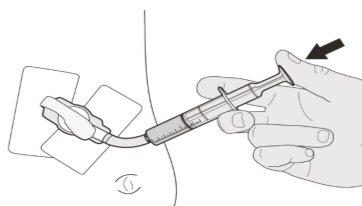
Figura C | Paso A2 Empuje el émbolo de la jeringa oral hasta el fondo para eliminar el aire de la jeringa oral (ver figura C). | |||||||
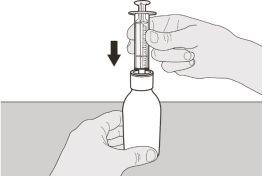
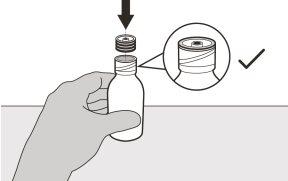
Figura D | Paso A3 Mantenga el frasco en vertical e inserte el extremo de la jeringa en el adaptador para el frasco (ver figura D). | |||||||
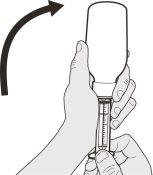
Figura E | Paso A4 Con cuidado, ponga el frasco boca abajo con el extremo de la jeringa firmemente insertado en el adaptador para el frasco (ver figura E). | |||||||
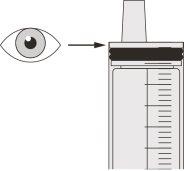
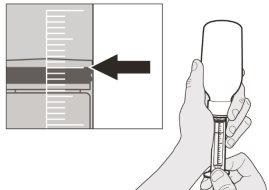
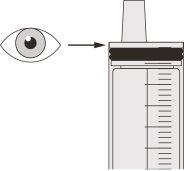
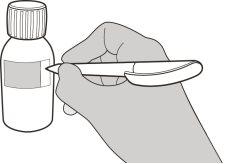
Figura F | Paso A5 Tire lentamente del émbolo para extraer la dosis de Evrysdi. La parte superior de la goma negra del émbolo debe alinearse con la marca de ml de la jeringa oral correspondiente a la dosis diaria (ver figura F). Una vez extraída la dosis correcta, mantenga el émbolo en su sitio para evitar que se mueva. | |||||||
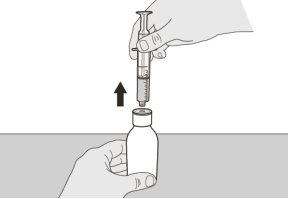
Figura G | Paso A6 Continúe manteniendo el émbolo en su sitio para que no se mueva.Deje la jeringa oral en el adaptador para el frasco y giré el frasco en posición vertical. Coloque el frasco en una superficie plana. Saque la jeringa oral del adaptador para el frasco tirando suavemente hacia arriba de la jeringa oral (ver figura G). | |||||||
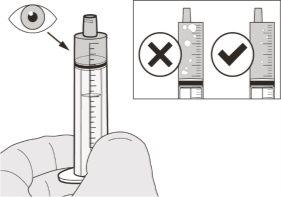
Figura H | Paso A7 Sujete la jeringa oral con el extremo de la jeringa mirando hacia arriba. Inspeccione el medicamento que hay dentro de la jeringa oral. Sihay burbujas grandes de aire en la jeringa oral (ver figura H) osiha extraído una dosis de Evrysdi incorrecta, inserte el extremo de la jeringa con firmeza en el adaptador para el frasco. Empuje el émbolo hasta el fondo para que el medicamento vuelva a introducirse en el frasco y repita desde el paso A4 al A7. Tome o administre Evrysdi inmediatamente después de extraerlo con la jeringa oral. Si no se toma en el plazo de 5minutos, deseche el medicamento de la jeringa oral y extraiga una nueva dosis. | |||||||
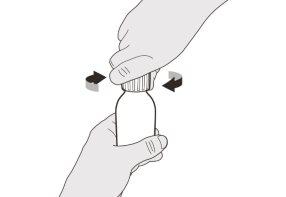
Figura I | Paso A8 Vuelva a poner el tapón en el frasco. Gire el tapón a la derecha (en el sentido de las agujas del reloj) para cerrar el frasco perfectamente (ver figura I). No saque del frasco el adaptador para el frasco. | |||||||
Si va a tomar la dosis de Evrysdi por la boca, siga las instrucciones que se dan en “B) Cómo tomar la dosis de Evrysdi por la boca”. Si va a tomar la dosis de Evrysdi mediante una sonda de gastrostomía, siga las instrucciones que se dan en “C) Cómo administrar la dosis de Evrysdi mediante una sonda de gastrostomía (sondaG)”. Si va a tomar la dosis de Evrysdi mediante una sonda nasogástrica, siga las instrucciones que se dan en “D) Cómo administrar la dosis de Evrysdi mediante una sonda nasogástrica (sondaNG)”. Las jeringas orales de Evrysdi están diseñadas específicamente para ser compatibles con el sistema ENFit®. Si la sonda de alimentación no es compatible con ENFit® puede necesitar un conector de transición ENFit® para conectar la jeringa de Evrysdi con la sonda G o la sonda NG. | ||||||||
| ||||||||
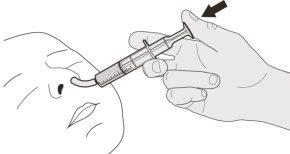
Permanezca sentado y erguido cuando tome la dosis de Evrysdi por la boca. | ||||||||
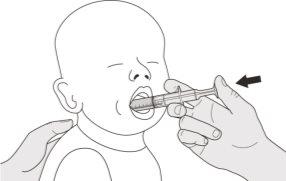
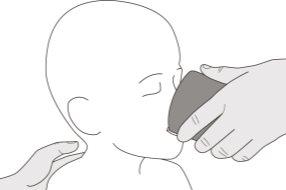
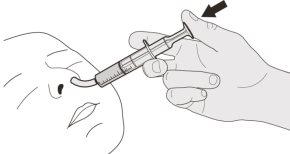
Figura J | Paso B1 Coloque la jeringa oral dentro de la boca con la punta junto a cualquiera de las mejillas. Lentamenteempuje el émbolo hasta el fondo para administrar la dosis completa de Evrysdi (ver figura J). Administrar Evrysdi en la parte posterior de la garganta o demasiado rápido puede provocar atragantamiento. | |||||||
Figura K | Paso B2 Compruebe que no queda medicamento en la jeringa oral (ver figura K). | |||||||
Figura L | Paso B3 Bebaun poco de agua inmediatamente después de tomar la dosis de Evrysdi (ver figura L). Vaya al pasoE para limpiar la jeringa. | |||||||
| ||||||||
Si va a administrar Evrysdi mediante una sonda de gastrostomía, pida a su médico o enfermero que le enseñe a inspeccionar la sonda de gastrostomía antes de administrar Evrysdi. | ||||||||
Figura M | Paso C1 Coloque el extremo de la jeringa oral dentro de la sonda de gastrostomía. Empuje lentamente el émbolo hasta el fondo para administrar la dosis completa de Evrysdi (ver figura M). | |||||||
Figura N | Paso C2 Compruebe que no queda medicamento en la jeringa oral (ver figura N). | |||||||
Figura O | Paso C3 Lave la sonda de gastrostomía con 10-20 ml de agua inmediatamente después de administrar la dosis de Evrysdi (ver figura O). Vaya al pasoE para limpiar la jeringa. | |||||||
| ||||||||
Si va a administrar Evrysdi mediante una sonda nasogástrica, pida a su médico o enfermero que le enseñe a inspeccionar la sonda nasogástrica antes de administrar Evrysdi. | ||||||||
Figura P | Paso D1 Coloque el extremo de la jeringa oral dentro de la sonda nasogástrica. Empuje lentamente el émbolo hasta el fondo para administrar la dosis completa de Evrysdi (ver figura P). | |||||||
Figura Q | Paso D2 Compruebe que no queda medicamento en la jeringa oral (ver figura Q). | |||||||
Figura R | Paso D3 Lave la sonda nasogástrica con 10-20 ml de agua inmediatamente después de administrar la dosis de Evrysdi (ver la figura D). Vaya al pasoE para limpiar la jeringa. | |||||||
| ||||||||
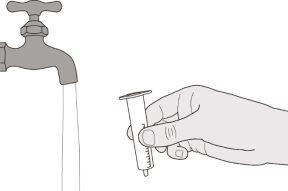
Figura S | Paso E1 Retire el émbolo de la jeringa oral. Enjuague bien el cuerpo de la jeringa oral con agua limpia (ver figura S). | |||||||
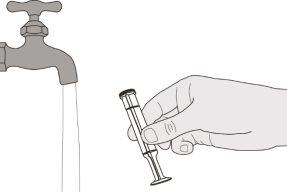
Figura T | Paso E2 Enjuague bien el émbolo con agua limpia (ver figura T). | |||||||
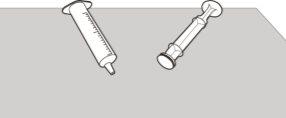
Figura U | Paso E3 Compruebe que el cuerpo y el émbolo de la jeringa oral están limpios. Coloque el cuerpo y el émbolo de la jeringa oral en una superficie limpia, en un lugar seguro, para que se sequen (ver figura U). Lávese las manos. Una vez secos, vuelva a montar el émbolo en el cuerpo de la jeringa oral y guarde la jeringa con el medicamento. |
Instrucciones de reconstitución
Evrysdi 0,75mg/ml
polvo para solución oral
risdiplam
Instrucciones de reconstitución
(SOLO PARA PROFESIONALES SANITARIOS [P. EJ, FARMACÉUTICOS])
Cada caja de Evrysdi contiene (ver figuraA):
|
Figura A |
Información importante sobre Evrysdi ? Evite inhalarel polvo de Evrysdi. ? Utilice guantes. ?Nolo use después de que haya pasado la fecha de caducidad del polvo. La fecha de caducidad del polvo está impresa en la etiqueta del frasco. ? Nodispense la solución reconstituida si la fecha de “Desechar después de” de la solución es posterior a la fecha de caducidad del polvo original. ? Evite el contactode la piel con el medicamento. Si el medicamento entra en contacto con la piel, lave la zona con agua y jabón. ? Nouse el medicamento si falta alguno de los suministros o están dañados. ? Utilice agua purificada o agua para preparaciones inyectables para reconstituir el medicamento. ? No añada jeringas orales distintas de las suministradas dentro del embalaje. | |
Conservación de Evrysdi ? Conserve el polvo (medicamento sin reconstituir) a temperatura ambiente y dentro del embalaje. ? Conserve la solución (medicamento reconstituido) en nevera (entre 2 °C y 8 °C) y dentro del embalaje en posición vertical. ? Conserve la solución oral en el frasco original y mantenga siempre el frasco en posición vertical con el tapón perfectamente cerrado. | |
Reconstitución | |
Figura B | Paso1 Golpee suavemente la parte inferior del frasco para soltar el polvo (ver figura B). |
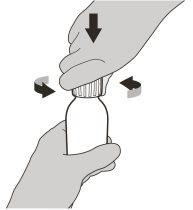
Figura C | Paso2 Retire el tapón presionando hacia abajo y luego girándolo a la izquierda (en sentido contrario a las agujas del reloj) (ver figura C). No deseche el tapón. |
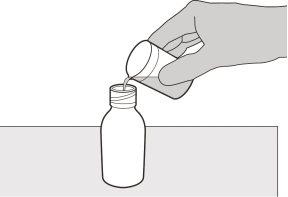
Figura D | Paso3 Vierta con cuidado 79 ml de agua purificada o agua para preparaciones inyectables en el frasco del medicamento (ver figura D). |
Figura E | Paso4 Sobre una mesa sujete el frasco del medicamento con una mano. Inserte el adaptador para el frasco en el orificio empujándolo hacia abajo con la otra mano. Asegúrese de que está completamente presionado contra el borde del frasco (ver figura E). |
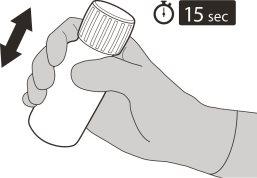
Figura F | Paso5 Vuelva a poner el tapón en el frasco. Gire el tapón a la derecha (en el sentido de las agujas del reloj) para cerrar el frasco. Asegúrese de que está perfectamente cerrado y agítelo bien durante 15 segundos (ver figura F). Espere 10 minutos. Debe haber obtenido una solución transparente. Posteriormente, vuelva a agitar bien durante 15 segundos. |
Figura G | Paso6 Calcule la fecha de “Desechar después de” en 64díasdespués de la reconstitución (Nota: el día de la reconstitución se cuenta como día 0. Por ejemplo, si la reconstitución tiene lugar el 1 de abril, la fecha de “Desechar después de” será el 4 de junio). Escriba la fecha de “Desechar después de”de la solución en la etiqueta del frasco (ver figura G) y en el embalaje. Vuelva a introducir el frasco en su embalaje original, con las jeringas (en bolsas), el prospecto y el folleto de Instrucciones de uso. Conserve el embalaje en la nevera (de 2 ºC a 8 ºC). |
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a EVRYSDI 0,75 mg/ml POLVO PARA SOLUCION ORALForma farmacéutica: COMPRIMIDO, 5 mgPrincipio activo: RisdiplamFabricante: Roche Registration GmbhRequiere recetaForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 8,86 mg/mlPrincipio activo: givinostatFabricante: Italfarmaco S.P.A.Requiere recetaForma farmacéutica: INYECTABLE, 20 mgPrincipio activo: Hialuronico acidoFabricante: Laboratorios Fidia Farmaceutica S.L.Requiere receta
Médicos online para EVRYSDI 0,75 mg/ml POLVO PARA SOLUCION ORAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de EVRYSDI 0,75 mg/ml POLVO PARA SOLUCION ORAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes