ENTYVIO 108 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA

Cómo usar ENTYVIO 108 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Entyvio 108mg solución inyectable en pluma precargada
vedolizumab
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas, aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Entyvio y para qué se utiliza
- Qué necesita saber antes de empezar a usar Entyvio
- Cómo usar Entyvio
- Posibles efectos adversos
- Conservación de Entyvio
- Contenido del envase e información adicional
1. Qué es Entyvio y para qué se utiliza
Qué es Entyvio
Entyvio contiene el principio activo “vedolizumab”. Vedolizumab pertenece a un grupo de medicamentos biológicos denominados anticuerpos monoclonales (Mab).
Cómo funciona Entyvio
Entyvio bloquea una proteína en la superficie de los glóbulos blancos (leucocitos) que causa la inflamación en la colitis ulcerosa y en la enfermedad de Crohn, de modo que se reduce la inflamación.
Para qué está indicado Entyvio
Entyvio se utiliza para tratar los signos y síntomas en adultos con:
- colitis ulcerosa activa de moderada a grave
- enfermedad de Crohn activa de moderada a grave.
Colitis ulcerosa
La colitis ulcerosa es una enfermedad que provoca la inflamación del intestino grueso. Si padece colitis ulcerosa se le administrarán primero otros medicamentos. Si no responde de manera satisfactoria o no tolera dichos medicamentos, su médico puede prescribirle Entyvio para reducir los signos y síntomas de la enfermedad.
Enfermedad de Crohn
La enfermedad de Crohn es una enfermedad que provoca la inflamación del aparato digestivo. Si padece enfermedad de Crohn se le administrarán primero otros medicamentos. Si no responde de manera satisfactoria o no tolera dichos medicamentos, su médico puede prescribirle Entyvio para reducir los signos y síntomas de la enfermedad.
2. Qué necesita saber antes de empezar a usar Entyvio
No use Entyvio
- si es alérgico a vedolizumab o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- si tiene una infección activa grave, por ejemplo, tuberculosis, septicemia, vómitos o diarrea graves (gastroenteritis) o infección del sistema nervioso.
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de usar Entyvio.
Informe a su médico, farmacéutico o enfermero inmediatamentecuando use este medicamento por primera vez, durante el tratamiento y en los periodos entre dosis:
- si experimenta visión doble, borrosa o pérdida de visión, dificultad en el habla, debilidad en un brazo o pierna, un cambio en su modo de caminar o problemas de equilibrio, entumecimiento persistente, disminución o pérdida de sensibilidad, confusión o pérdida de memoria. Todos estos síntomas podrían corresponder a una complicación cerebral grave y potencialmente mortalconocida como leucoencefalopatía multifocal progresiva (LMP).
- si tiene una infección, o cree que tiene una infección ‑los signos pueden ser escalofríos, temblores, tos persistente o fiebre alta‑. Algunas infecciones pueden ser graves e incluso potencialmente mortales si no se tratan.
- si experimenta signos de una reacción alérgicacomo silbidos, dificultad para respirar, habones, picores, hinchazón o mareos. Para información más detallada, consulte el apartado sobre reacciones alérgicas en la sección 4.
- si va a recibir alguna vacunao la ha recibido recientemente. Entyvio puede afectar al modo en que usted responde a una vacuna.
- si tiene cáncer, dígaselo a su médico. Su médico tendrá que decidir si es posible administrarle Entyvio.
- si no se siente nada mejor, dado que el vedolizumab puede tardar hasta 14 semanas en actuar en algunos pacientes con enfermedad de Crohn muy activa.
Niños y adolescentes
Entyvio no está recomendado para su uso en niños y adolescentes (menores de 18 años de edad) debido a la falta de información sobre la utilización de este medicamento en este grupo de edad.
Otros medicamentos y Entyvio
Informe a su médico, farmacéutico o enfermero si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
- Entyvio no se debe administrar junto con otros medicamentos biológicos supresores del sistema inmunitario, ya que se desconocen los efectos que se pueden producir.
Informe a su médico si con anterioridad se le ha administrado:
- natalizumab (un medicamento indicado para la esclerosis múltiple) o
- rituximab (un medicamento indicado para ciertos tipos de cáncer y la artritis reumatoide).
Su médico tendrá que decidir si es posible administrarle Entyvio.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
Embarazo
Se desconocen los efectos de Entyvio en mujeres embarazadas. Por lo tanto, no se recomienda utilizar este medicamento durante el embarazo. Usted y su médico deben decidir que el beneficio para usted es claramente superior al riesgo potencial para usted y su bebé.
Si es una mujer en edad fértil, se recomienda que evite quedarse embarazada durante el uso de Entyvio. Debe utilizar métodos anticonceptivos adecuados durante el tratamiento y durante al menos 4.5 meses después de recibir la última dosis.
Lactancia
Informe a su médico si está en periodo de lactancia o tiene intención de estarlo. Entyvio pasa a la leche materna. No existe suficiente información relativa a los efectos que ello puede tener en su bebé y en la producción de leche. Se debe decidir si interrumpir la lactancia o el tratamiento con Entyvio, para lo cual será necesario evaluar los beneficios de la lactancia para su bebé y los beneficios del tratamiento para usted.
Conducción y uso de máquinas
Los efectos de este medicamento sobre la capacidad para conducir y utilizar máquinas o herramientas son pequeños. Un número reducido de pacientes se han sentido mareados después de recibir Entyvio. Si se siente mareado, no conduzca ni utilice herramientas o máquinas.
Entyvio 108mg solución inyectable contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por unidad de dosis; esto es, esencialmente “exento de sodio”.
3. Cómo usar Entyvio
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consúlteles de nuevo.
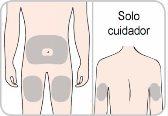
Usted o su cuidador recibirán formación sobre el uso de las inyecciones bajo la piel (las inyecciones subcutáneas) de Entyvio.
Cuánto Entyvio se le administrará
El tratamiento con Entyvio es el mismo para la colitis ulcerosa y para la enfermedad de Crohn.
La dosis recomendada es de 108 mg de Entyvio administrados mediante inyección subcutáneo una vez cada 2 semanas.
- Al inicio del tratamiento, su médico le administrará las dosis iniciales de Entyvio mediante un sistema de perfusión con goteo en una de las venas del brazo (perfusión intravenosa) durante unos 30 minutos.
- Tras como mínimo 2 perfusiones intravenosas, podrá iniciar el tratamiento con Entyvio mediante inyección subcutánea. La primera inyección subcutánea se administra en el momento de la siguiente perfusión intravenosa programada, y cada 2 semanas a partir de entonces.
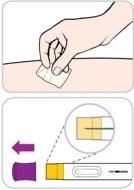
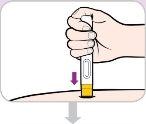
Cómo inyectar Entyvio
Usted mismo pueden administrarse las inyecciones subcutáneas, o puede hacerlo un cuidador, tras recibir formación sobre ello. Al final de este prospecto encontrará instrucciones sobre cómo proceder a la inyección subcutánea.
Si olvidó u omitió una inyección de Entyvio
Si olvida u omite una dosis, inyecte la siguiente dosis tan pronto como le sea posible y, a continuación, cada 2 semanas.
Si interrumpe el tratamiento con Entyvio
No debe dejar de usar Entyvio sin hablar antes con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos graves
Informe a su médico inmediatamentesi nota alguno de los siguientes síntomas:
- reacciones alérgicas (pueden afectar hasta 1 de cada 100 pacientes), con signos como silbidos o dificultad para respirar, habones, picor de la piel, hinchazón, sensación de malestar, dolor en el lugar de perfusión, enrojecimiento de la piel
- infecciones (pueden afectar hasta 1 de cada 10 pacientes), con signos como escalofríos o temblores, fiebre alta o erupciones
Otros efectos adversos
Informe a su médico lo antes posiblesi nota alguno de los siguientes síntomas:
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 pacientes)
- resfriado común
- dolor en las articulaciones
- dolor de cabeza
Efectos adversos frecuentes(pueden afectar hasta 1 de cada 10 pacientes)
- neumonía
- infección bacteriana del intestino grueso por Clostridium difficile
- fiebre
- infección respiratoria
- cambios en el funcionamiento del hígado, aumento de las enzimas hepáticas (se muestran en los análisis de sangre)
- cansancio
- tos
- gripe
- dolor de espalda
- dolor de garganta
- sinusitis
- picor/prurito
- erupción y enrojecimiento
- dolor en las extremidades
- calambres musculares
- debilidad muscular
- infección de garganta
- gripe estomacal
- infección anal
- dolor anal
- heces duras
- estómago hinchado
- flatulencias
- tensión arterial alta
- entumecimiento u hormigueo
- ardor de estómago
- hemorroides
- nariz taponada
- eccema
- sudoración nocturna
- acné (espinillas)
- reacciones en la zona de la inyección (dolor, hinchazón, eritema o picor)
- herpes zóster
Efectos adversos poco frecuentes(pueden afectar hasta 1 de cada 100 pacientes)
- enrojecimiento y sensibilidad de los folículos pilosos
- infección de boca y garganta por levaduras
- infección vaginal
- visión borrosa (pérdida de la agudeza visual)
Efectos adversos muy raros(pueden afectar hasta 1 de cada 10.000 pacientes)
- reacción alérgica repentina y grave que puede causar dificultad para respirar, inflamación, aceleración del ritmo cardíaco, sudoración, bajada de tensión arterial, mareo, pérdida del conocimiento y desmayo (reacción anafiláctica y shock anafiláctico)
- inflamación del hígado (hepatitis). Los signos y síntomas de hepatitis pueden incluir pruebas de función hepática anormales, amarilleamiento de los ojos o la piel (ictericia), dolor en la parte derecha del área del estómago o cardenales
Frecuencia no conocida(la frecuencia no puede estimarse a partir de los datos disponibles)
- enfermedad pulmonar que provoca dificultad para respirar (enfermedad pulmonar intersticial)
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Entyvio
- Mantener este medicamento fuera de la vista y del alcance de los niños.
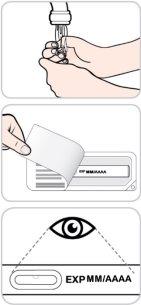
- No utilice este medicamento después de la fecha de caducidad que aparece en el estuche, y en la etiqueta, después de "CAD". La fecha de caducidad es el último día del mes que se indica.
- Entyvio es para un solo uso.
- Conservar en nevera (entre 2 °C y 8 °C). Conservar la(s) pluma(s) precargada(s) en el estuche original para protegerlas de la luz. Si fuese necesario, una única pluma precargada puede conservarse fuera de la nevera protegida de la luz a temperatura ambiente (hasta 25 °C) durante 7 días, como máximo. No utilice la pluma si lleva más de 7 días fuera de la nevera.
- No congelar. No exponer directamente a la luz del sol.
- No utilizar este medicamento si se observa presencia de partículas en el líquido o decoloración (debe ser incolora o presentar una tonalidad amarillenta) antes de la administración.
- Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Entyvio
- El principio activoes vedolizumab. Cada pluma precargada contiene 108 mg de vedolizumab.
- Los demás componentesson ácido cítrico monohidratado, citrato sódico dihidratado, L‑histidina, monohidrocloruro de L‑histidina, hidrocloruro de L‑arginina, polisorbato 80 y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
- Entyvio es una solución inyectable incolora o de color amarillento que se proporciona en una pluma precargada de vidrio con un mecanismo de seguridad automático que protege y bloquea la aguja tras extraer el dispositivo de la zona de inyección
- Entyvio está disponible en estuches con 1 o 2 plumas precargadas y en envases múltiples con 6 (6 × 1) plumas precargadas. Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Takeda Pharma A/S
Delta Park 45
2665 Vallensbaek Strand
Dinamarca
Responsable de la fabricación
Takeda Austria GmbH
St. Peter‑Straβe 25
A‑4020 Linz
Austria
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Takeda Belgium NV Tél./Tel.: +32 2 464 06 11 | Lietuva Takeda, UAB Tel.: +370 521 09 070 |
| Luxembourg/Luxemburg Takeda Belgium NV Tél./Tel.: +32 2 464 06 11 |
Ceská republika Takeda Pharmaceuticals Czech Republic s.r.o. Tel: + 420 234 722 722 | Magyarország Takeda Pharma Kft. Tel.: +361 2707030 |
Danmark Takeda Pharma A/S Tlf: +45 46 77 10 10 | Malta Drugsales Ltd Tel.: +356 2141 9070 |
Deutschland Takeda GmbH Tel.: +49 (0) 800 825 3325 | Nederland Takeda Nederland B.V. Tel.: +31 20 203 5492 |
Eesti Takeda Pharma OÜ Tel.: +372 6177 669 | Norge Takeda AS Tlf.: +47 800 800 30 |
Ελλ?δα TAKEDA ΕΛΛΑΣ Α.Ε. Τηλ.: +30 210 6387800 | Österreich Takeda Pharma Ges.m.b.H. Tel.: +43 (0) 800 20 80 50 |
España Takeda Farmacéutica España, S.A. Tel.: +34 917 90 42 22 | Polska Takeda Pharma Sp. z o.o. Tel.: +48223062447 |
France Takeda France SAS Tel.: +33 1 40 67 33 00 | Portugal Takeda Farmacêuticos Portugal, Lda. Tel.: +351 21 120 1457 |
Hrvatska Takeda Pharmaceuticals Croatia d.o.o. Tel: +385 1 377 88 96 | România Takeda Pharmaceuticals SRL Tel.: +40 21 335 03 91 |
Ireland Takeda Products Ireland Ltd. Tel.: 1800 937 970 | Slovenija Takeda Pharmaceuticals farmacevtska družba d.o.o. Tel.: +386 (0) 59 082 480 |
Ísland Vistor hf. Simi: +354 535 7000 | Slovenská republika Takeda Pharmaceuticals Slovakia s.r.o. Tel.: +421 (2) 20 602 600 |
Italia Takeda Italia S.p.A Tel.: +39 06 502601 | Suomi/Finland Takeda Oy Puh./Tel.: 0800 774 051 |
Κ?προς A.POTAMITIS MEDICARE LTD Τηλ: +357 22583333 | Sverige Takeda Pharma AB Tel.: 020 795 079 |
Latvija Takeda Latvia SIA Tel.: +371 67840082 | United Kingdom (Northern Ireland) Takeda UK Ltd Tel.: +44 (0) 3333 000 181 |
Fecha de la última revisión de este prospecto: 03/2025
Otras fuentes de información
Este prospecto está disponible en formato adecuado para pacientes ciegos o con visión reducida y puede solicitarse al representante local del titular de la autorización de comercialización.
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
-----------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
Trazabilidad
Con objeto de mejorar la trazabilidad de los medicamentos biológicos, el nombre y el número de lote del medicamento administrado deben estar claramente registrados.
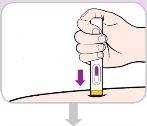
Instrucciones de uso:
Lea y siga estas instrucciones antes de proceder a la inyección. Su médico, enfermero o farmacéutico deben mostrarle cómo utilizar la pluma precargada de Entyvio antes de usarla por primera vez.
Su pluma precargada de Entyvio de un solo uso
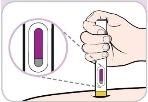
Antes de usar | |||
Tapón de color morado | Ventana de visualización | ||
|
Después de usar | |||
Protección de aguja de color amarillo | Ventana de visualización (inyección finalizada) | ||
|
| |
| Espere 30minutos
|
| |
|
|
| |
|
|
| |
| PRESIONAR
SOSTENER (contar hasta 10)
CONFIRMAR
|
| |
|
|
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ENTYVIO 108 MG SOLUCION INYECTABLE EN PLUMA PRECARGADAForma farmacéutica: INYECTABLE, 108 mgPrincipio activo: VedolizumabFabricante: Takeda Pharma A/SRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, 300 mgPrincipio activo: VedolizumabFabricante: Takeda Pharma A/SRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, 120 mg (80 mg/kg) belimumabPrincipio activo: BelimumabFabricante: Glaxosmithkline (Ireland) LimitedRequiere receta
Médicos online para ENTYVIO 108 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ENTYVIO 108 MG SOLUCION INYECTABLE EN PLUMA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes