
DUKORAL SUSPENSION AND EFFERVSCENT GRANULES FOR ORAL SUSPENSION

How to use DUKORAL SUSPENSION AND EFFERVSCENT GRANULES FOR ORAL SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
DUKORAL suspension and effervescent granule for oral suspension
Cholera vaccine (inactivated, oral)
Read all of this leaflet carefully before you start using this vaccine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This vaccine has been prescribed for you, do not pass it on to others.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
- Make sure to mix the vaccine with the buffer solution as described in this leaflet. See section 3.
Contents of thepackage leaflet
- What is Dukoral and what is it used for
- What you need to know before you start using Dukoral
- How to use Dukoral
- Possible side effects
- Storage of Dukoral
- Contents of the pack and further information
1. What is Dukoral and what is it used for
Dukoral is an oral cholera vaccine that stimulates the intestinal immune defenses. The vaccine protects adults and children from 2 years of age against cholera.
Dukoral makes the body produce its own protection against cholera. After receiving the vaccine, the body will create substances called antibodies that fight the toxin and cholera bacteria that cause diarrhea.
2. What you need to know before you start using Dukoral
Do not use Dukoral
? if you are allergic to any of the components of the vaccine (listed in section 6) or to formaldehyde.
? if you have an acute gastric disorder or an infection accompanied by fever (vaccination should be postponed).
Warnings and precautions
Talk to your doctor before starting to take Dukoral
? if you are receiving treatment that affects your immune system
? if you have an immune system disease (including HIV infection).
The vaccine may provide a lower level of protection than that which occurs in people without immune system impairment.
The vaccine does not provide complete protection and it is important to follow dietary and hygiene recommendations to avoid contracting diseases that cause diarrhea.
Children
Do not administer this vaccine to children under 2 years of age as protection has not been studied in this group.
Use ofDukoral withother medicines
Tell your doctor if you are taking, have recently taken, or might take any other medicine.
Do not take any other medicine from one hour before to one hour after administration of the vaccine.
Use of Dukoral with food and drinks
Avoid ingestion of food and drinks from one hour before to one hour after administration of the vaccine.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before taking the vaccine.
Driving and using machines
There is no reason to suspect that Dukoral affects the ability to drive and use machines.
Dukoralcontains sodium
Dukoral contains approximately 1.1 g of sodium per dose, which should be taken into account in patients with low-sodium diets.
3. How to use Dukoral
Follow the instructions for administration of the medicine contained in this leaflet or as indicated by your doctor. In case of doubt, consult your doctor again.
Adults and children from 6 years of age:The primary vaccination consists of two doses orally (by mouth) separated by a minimum interval of one week (up to six weeks).
- Take the first dose no later than two weeks before starting your trip.
- Take the second dose at least one week after the first dose and at least one week before your trip.
It takes approximately one week for protection to begin after the last dose.
To achieve continued protection, revaccination is recommended after two years. If it has been less than two years since your last dose of the vaccine, a single dose will renew your protection. If it has been more than two years since your last dose of the vaccine, primary vaccination should be repeated (2 doses).
Children from 2 to less than 6 years of age:The primary vaccination consists of three doses taken orally (by mouth) separated by a minimum interval of one week (up to six weeks). Only half of the buffer solution should be mixed with the vaccine.
- Administer the first dose to the child no later than three weeks before starting your trip.
- Administer the second dose to the child at least one week after the first dose.
- Administer the third dose to the child at least one week after the second dose and at least one week before your trip.
It takes approximately one week for protection to begin after the last dose. To achieve continued protection, revaccination is recommended after six months. If it has been less than six months since the last dose of the vaccine, a single dose will renew protection. If it has been more than six months since the last vaccination, primary vaccination should be repeated (3 doses).
The suspension is a white suspension that is supplied in a glass vial for single dose. Each vial comes with a sachet containing a white, effervescent granule of sodium bicarbonate. The effervescent granule should be dissolved in a glass of cold water, and the resulting buffer solution should be mixed with the suspension. It is important to use the buffer solution, as it protects the vaccine against gastric acid.
Drink the entire mixture within 2 hours after mixing with the buffer solution.
Instructions for use:
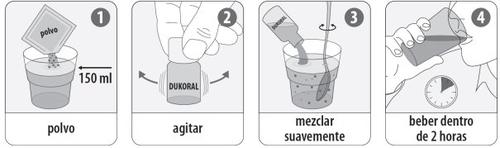
- To prepare the buffer solution, dissolve the effervescent granule in a glass of cold water (approx. 150 ml) by gently stirring.
Do not use any other liquid.
For children from 2 to less than 6 years:discard half of the buffer solution.
- Shake the Dukoral suspension vial (1 vial = 1 dose).
- Pour the contents of the Dukoral suspension vial into the glass of buffer solution (see 1). Mix by gently stirring.
- Drink the entire mixture within 2 hours. Avoid ingestion of food and drinks from one hour before to one hour after drinking the mixture.
If you take more Dukoral than you should
If you take the doses with a time interval of less than one week, contact your doctor, pharmacist, or nurse.
As each vial of Dukoral contains only one dose, it is unlikely that an overdose will occur.
If you have taken more than one dose at a time, contact your doctor, pharmacist, or nurse.
If you forget to take Dukoral
You can take the second dose of Dukoral up to 6 weeks after the first dose (children from 2 to less than 6 years of age have to take 3 doses). If more than 6 weeks have passed, contact your doctor, pharmacist, or nurse.
If you have any further questions on the use of this vaccine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, Dukoral can cause side effects, although not everybody gets them.
Contact a doctor immediately if you experience the following serious side effects:
- Severe diarrhea with loss of body water
- Severe allergic reactions that cause swelling of the face or throat and difficulty breathing
Other side effects:
Uncommon side effects (may affect up to 1 in 100 people)
- Diarrhea, stomach pain, abdominal cramps, abdominal rumbling, abdominal swelling, gas in the stomach, and general abdominal discomfort
- Headache
Rare side effects (may affect up to 1 in 1,000 people)
- Fever
- General malaise, feeling of dizziness
- Nausea, vomiting, loss or decreased appetite
- Swelling, inflammation inside the nose, and cough
Very rare side effects (may affect up to 1 in 10,000 people)
- Rash
- Sore throat, decreased sense of taste
- Fatigue/feeling of tiredness
- Sweating, chills
- Joint pain
- Difficulty sleeping
Other side effects (frequency cannot be estimated from the available data)
- Pseudo-flu symptoms, respiratory symptoms, chills, general pain, weakness
- Hives, itching
- Swelling of the lymph nodes
- Numbness or tingling
- High blood pressure
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Dukoral
Keep this medicine out of the sight and reach of children.
Do not use Dukoral after the expiry date which is stated on the carton after EXP. The expiry date is the last day of the month stated.
Store in a refrigerator (between 2°C and 8°C). Do not freeze.
The product from the vial and sachet unopened, stored in the outer carton, remains stable at temperatures not above 25°C for a period of 14 days. At the end of this period, the product should be used or discarded.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and further information
Composition of Dukoral
- The active substances are: 31.25x10^9 bacteria* of the following strains of V. choleraeO1: classical biotype Inaba (inactivated by heat), El Tor biotype Inaba (inactivated with formalin), classical biotype Ogawa (inactivated by heat), classical biotype Ogawa (inactivated with formalin). Recombinant cholera toxin B subunit (TCBr), 1 mg.
*bacterial content prior to inactivation
- The other components of the suspension that contains the vaccine are sodium dihydrogen phosphate, disodium hydrogen phosphate, sodium chloride, and water for injections.
- The effervescent granule contains sodium bicarbonate, citric acid, sodium carbonate, sodium saccharin, sodium citrate, and raspberry flavor.
Appearance of the product and contents of the pack
Dukoral is presented as a suspension and effervescent granule for oral suspension. The suspension is a white suspension that is presented in a vial. The effervescent granule is white with a raspberry flavor and is supplied in a sachet.
Dukoral is presented in packs of 1, 2, and 20 doses. Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer
Valneva Sweden AB, 105 21 Stockholm, Sweden.
Date of last revision ofthis leaflet:MM/AAAA
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
On the European Medicines Agency website, you can find this leaflet in all languages of the European Union/European Economic Area.
- Country of registration
- Average pharmacy price49.39 EUR
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DUKORAL SUSPENSION AND EFFERVSCENT GRANULES FOR ORAL SUSPENSIONDosage form: ORAL SOLUTION/SUSPENSION, 4x10^8 - 2x10^9 CFU/doseActive substance: cholera, live attenuatedManufacturer: Bavarian Nordic A/SPrescription requiredDosage form: INJECTABLE, 0.5 ml single doseActive substance: meningococcus B, multicomponent vaccineManufacturer: Glaxosmithkline Vaccines S.R.L.Prescription requiredDosage form: INJECTABLE, 0.5 ml single doseActive substance: meningococcus B, multicomponent vaccineManufacturer: Glaxosmithkline Vaccines S.R.L.Prescription required
Online doctors for DUKORAL SUSPENSION AND EFFERVSCENT GRANULES FOR ORAL SUSPENSION
Discuss questions about DUKORAL SUSPENSION AND EFFERVSCENT GRANULES FOR ORAL SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















