
COLIRCUSI DEXAMETHASONE 1 mg/ml eye drops solution


How to use COLIRCUSI DEXAMETHASONE 1 mg/ml eye drops solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
COLIRCUSI DEXAMETASONA 1 mg/ml eye drops, solution
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What COLIRCUSI DEXAMETASONA is and what it is used for
- What you need to know before using COLIRCUSI DEXAMETASONA
- How to use COLIRCUSI DEXAMETASONA
- Possible side effects
- Storage of COLIRCUSI DEXAMETASONA
- Package contents and further information
1. What is Colicursi Dexametasona and what is it used for
It is an eye drop solution that contains the active ingredient dexametasona phosphate sodium, a potent corticosteroid with anti-inflammatory and anti-allergic properties, which reduces the inflammatory response caused by allergic, mechanical, or chemical agents.
Colircusi Dexametasona is indicated for the treatment of non-infectious eye inflammations that respond to corticosteroids, which may affect the conjunctiva (the transparent membrane that covers the eye), the cornea, or the anterior pole of the eye. Spring conjunctivitis and allergic conjunctivitis. Superficial (episcleritis) and deep (scleritis) eye inflammations. Inflammations of the iris (iritis), ciliary body (ciclitis), or combined (iridociclitis) inflammations.
2. What you need to know before using Colicursi Dexametasona
Do not use Colircusi Dexametasona:
- If you are allergic to dexametasona or any other component of this medication (listed in section 6).
- If you have or think you have:
- Untreated bacterial eye infection.
- Corneal inflammation (keratitis) caused by herpes simplex or any other viral eye infection, such as smallpox or chickenpox.
- Tuberculosis that affects the eye.
- Fungal (fungal) eye diseases or untreated parasitic eye infections.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Colircusi Dexametasona.
- Use this medication only in your eye(s).
- This medication may increase eye pressure, especially if you already have glaucoma or high eye pressure, or a family history, so you should use it under medical supervision.
- If you use this medication for a long time, you may:
- Develop high eye pressure and/or glaucoma (with optic nerve damage and decreased visual acuity). You should regularly check your eye pressure while using this medication. Consult your doctor if you have any doubts. The risk of increased intraocular pressure and/or cataract formation induced by corticosteroids is higher in prone patients (e.g., diabetes).
- Develop cataracts. You should visit your doctor frequently.
- Develop Cushing's syndrome and/or suppression of adrenal gland function because the medication reaches the bloodstream. Consult your doctor if you experience swelling and weight gain around the trunk and face, as these are usually the first manifestations of Cushing's syndrome. Adrenal gland function suppression may occur after interrupting intensive or long-term treatment with Colircusi Dexametasona. Consult your doctor before interrupting treatment on your own. These risks are especially important in children and patients treated with a medication called ritonavir or cobicistat.
- If your symptoms worsen or return suddenly, please contact your doctor. You may become more susceptible to eye infections with the use of this medication. Corticosteroids can also mask the signs of an infection or worsen it, especially with prolonged use in purulent eye infections.
- If you already have a bacterial eye infection, you should consult your doctor about your treatment.
- Prolonged use of corticosteroids in the eye may produce fungal corneal infections. If this occurs, treatment should be discontinued. The use of corticosteroids in the eye in excessive doses can delay the healing of eye wounds. It is also known that ophthalmic non-steroidal anti-inflammatory drugs (NSAIDs) slow down or delay healing (see "Other medications and Colircusi Dexametasona"). If you suffer from a disorder that causes thinning of the eye tissue (the outermost layers of the eye), the use of this medication may cause corneal perforation.
- Contact your doctor if you experience blurred vision or other visual disturbances.
If you wear contact lenses:
- Wearing contact lenses (hard or soft) is not recommended during the treatment of an eye inflammation.
Children
The safety and efficacy of this medication have not been established in children. Therefore, its use is not recommended in children.
The possible increase in intraocular pressure associated with prolonged use of this medication is especially important in pediatric patients; the risk of corticosteroid-induced ocular hypertension may be higher in children and occur earlier than in adults.
Other medications and Colircusi Dexametasona
Inform your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
Inform your doctor if you are using ophthalmic NSAIDs. The concomitant use of steroids and ophthalmic NSAIDs can increase corneal healing problems.
Inform your doctor if you are using ritonavir or cobicistat, as they may increase the amount of dexametasona in the blood.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
The use of Colircusi Dexametasona is not recommended during pregnancy.
If you are breastfeeding, you should decide whether to interrupt breastfeeding or interrupt treatment with this medication, considering the benefit of breastfeeding for the child and the benefit of treatment for the mother.
Driving and using machines
You may notice that your vision becomes blurred for a while after applying the eye drops. Do not drive or use machines until this effect has disappeared.
Colircusi Dexametasona contains benzalkonium chloride
This medication may cause eye irritation because it contains benzalkonium chloride.
Avoid contact with soft contact lenses.
Remove contact lenses before application and wait at least 15 minutes before putting them back on.
It changes the color of soft contact lenses.
3. How to use Colicursi Dexametasona
Follow the administration instructions of this medication exactly as indicated by your doctor or pharmacist. If you have any doubts, consult your doctor or pharmacist again.
Ophthalmic route (in the eye(s)).
The recommended dose is:
Adults:
One or two drops in the affected eye(s) three times a day.
When a satisfactory response is observed after 3-4 days, the frequency of administration can be gradually reduced to once a day.
The maximum recommended treatment duration is 14 days, unless your doctor has given you different instructions.
Recommendations for use:
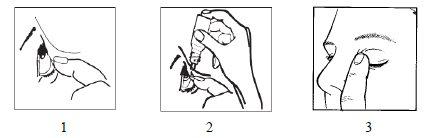
- Wash your hands.
- Take the bottle (dropper).
- After opening the bottle for the first time, remove the plastic ring from the seal if it is loose.
- Hold the bottle, upside down, between your fingers.
- Tilt your head back. Gently separate the eyelid from the eye with one finger until a pocket forms between the eyelid and your eye, where the drop should fall (figure 1).
- Bring the tip of the bottle close to the eye. You may find it helpful to use a mirror.
- Do not touch the eye or eyelid, or surrounding areas, with the dropper. The drops may become contaminated.
- Gently press the base of the bottle with your index finger to release one drop at a time (figure 2).
- After using the eye drops, press the edge of the eye near the nose with your finger. This helps prevent the medication from entering the rest of the body (figure 3).
- If you are applying drops to both eyes, repeat all the previous steps with the other eye.
- Close the bottle tightly immediately after use.
If a drop falls outside the eye, try again.
If you are using other ophthalmic medications, wait at least 5 minutes between the administration of this eye drop and other ophthalmic medications. Ophthalmic ointments should be administered last.
If you use more Colircusi Dexametasona than you should
An overdose in the eyes can be eliminated by rinsing the eyes with warm water. Do not apply more drops until the next dose.
In case of overdose or accidental ingestion, consult your doctor, pharmacist, or call the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount used.
If you forget to use Colircusi Dexametasona
Do not apply a double dose to make up for forgotten doses.
Apply a single dose as soon as you remember and continue with the next scheduled dose. However, if it is almost time for the next dose, do not apply the missed dose and continue with the next dose of your regular regimen.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
Side effects are classified by frequency, which is defined as: very common (may affect more than 1 in 10 people); common (may affect up to 1 in 10 people); uncommon (may affect up to 1 in 100 people); rare (may affect up to 1 in 1,000 people); very rare (may affect up to 1 in 10,000 people); frequency not known (cannot be estimated from available data).
The following side effects have been reported with this medication:
Common side effects:
- Eye effects: discomfort in the eye(s).
Uncommon side effects:
- Eye effects: inflammation of the eye surface (keratitis), conjunctivitis, dry eye, corneal spot, sensitivity to light (photophobia), blurred vision, abnormal sensation in the eye, tearing, eyelid crusts, itching, irritation, or redness in the eye.
- General effects: alteration of taste (bad taste).
Frequency not known:
- Eye effects: glaucoma (increased eye pressure with decreased visual acuity), corneal ulcer, increased pressure in the eye(s), reduced vision, corneal damage, eyelid drooping, eye pain, increased pupil size.
- General effects: hypersensitivity (allergy), dizziness, headache.
- Hormonal problems: excessive body hair growth (particularly in women), muscle weakness and wasting, purple striae on the body skin, increased blood pressure, irregular or absent menstrual periods, changes in body protein and calcium levels, delayed growth in children and adolescents, and swelling and weight gain in the body and face (Cushing's syndrome) (see section 2, "Warnings and precautions").
Prolonged use of corticosteroids in the eyes can also cause:
- Increased eye pressure with optic nerve damage and decreased visual acuity.
- Cataract formation.
- Delayed corneal healing.
- In diseases that cause thinning of the eye tissue, there is a higher risk of perforation.
Corticosteroids can reduce resistance to eye infections, favoring their occurrence.
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use: https://www.notificaRAM.es.By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Colicursi Dexametasona
Keep this medication out of the sight and reach of children.
Do not store above 25°C.
Do not use this medication after the expiration date shown on the bottle and carton after EXP. The expiration date is the last day of the month indicated.
To avoid infections, you should discard the bottle 4 weeks after opening it for the first time.
Write the opening date of the bottle in the space provided on the carton.
Medications should not be disposed of in wastewater or household waste. Deposit the packaging and medications you no longer need in the SIGRE collection point at the pharmacy. If you have any doubts, ask your pharmacist how to dispose of the packaging and medications you no longer need. This will help protect the environment.
6. Package contents and further information
Composition of Oftalmolosa Cusi Dexametasona
- The active ingredient is dexametasona phosphate sodium. Each gram of ointment contains 0.5 mg of dexametasona phosphate sodium (0.05%).
- The other ingredients are cholesterol, liquid paraffin, and white petrolatum.
Appearance of the product and package contents
Oftalmolosa Cusi Dexametasona is a white ophthalmic ointment.
It is presented in an aluminum tube with a polyethylene cap containing 3 g of ointment.
Marketing authorization holder
Fidia Farmaceutici S.p.A.
Via Ponte della Fabbrica, 3/A - 35031 Abano Terme – Italy
Manufacturer
Siegfried El Masnou S.A.
C/ Camil Fabra, 58
08320 El Masnou – Barcelona, Spain
Local representative
Laboratorios Fidia Farmacéutica S.L.U.
Parque Empresarial de la Moraleja - Edificio Torona
Avenida de Europa, 24 - Edificio A - 1 B
Alcobendas 28108 - Madrid, Spain
Date of the last revision of this package leaflet:November 2016.
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Average pharmacy price2.9 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to COLIRCUSI DEXAMETHASONE 1 mg/ml eye drops solutionDosage form: EYEDROP, 1 mg/mlActive substance: dexamethasoneManufacturer: Pharmaselect International Beteiligungs GmbhPrescription requiredDosage form: EYEDROP, 1mg/mlActive substance: dexamethasoneManufacturer: Laboratoires TheaPrescription requiredDosage form: EYEDROP, 1 mg/mlActive substance: dexamethasoneManufacturer: Brill Pharma S.L.Prescription required
Online doctors for COLIRCUSI DEXAMETHASONE 1 mg/ml eye drops solution
Discuss questions about COLIRCUSI DEXAMETHASONE 1 mg/ml eye drops solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















