
ANAMAP 25 mg/g + 25 mg/g CREAM

How to use ANAMAP 25 mg/g + 25 mg/g CREAM
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the patient
Anamap25 mg/g + 25 mg/g cream
Lidocaine + Prilocaine
Read the entire leaflet carefully before starting to take this medication, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any doubts, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are side effects not listed in this leaflet. See section 4.
Contents of the leaflet
- What is Anamap and what is it used for
- What you need to know before starting to use Anamap
- How to use Anamap
- Possible side effects
- Storage of Anamap
- Package contents and additional information
1. What is Anamap and what is it used for
Anamap contains two active ingredients called lidocaine and prilocaine. Both belong to the group of local anesthetic medications.
Anamap works by numbing the skin surface for a short time. It is applied to the skin before certain medical procedures. It helps to suppress pain in the skin; however, you may still perceive sensations such as pressure and contact.
Adults, adolescents, and children:
It can be used to anesthetize the skin before:
- Inserting a needle (e.g., for an injection or blood test).
- Minor skin surgeries.
Adults and adolescents
It can also be used:
- To numb the genital area before:
- An injection.
- Medical procedures such as wart removal.
The application of Anamap in the genital area must be performed by a doctor or nurse.
Adults:
It can also be used to numb the skin before:
- Cleaning or removing damaged skin from leg ulcers.
For any other purpose other than application to intact skin, the medication should only be used under the recommendation of a doctor, nurse, or pharmacist.
2. What you need to know before starting to use Anamap
Do not use Anamap:
- If you are allergic to lidocaine or prilocaine, other similar local anesthetics, or any of the other components of this medication (listed in section 6).
Warnings and precautions
Consult your doctor, pharmacist, or nurse before using Anamap:
- If you or your child have a rare hereditary blood disorder called glucose-6-phosphate dehydrogenase deficiency.
- If you or your child have a blood pigmentation problem called methemoglobinemia.
- Do not use Anamap on areas with skin rash, cuts, scratches, or other open wounds, except for a leg ulcer. If any of these problems appear, consult your doctor, pharmacist, or nurse before using the cream.
- If you or your child have a skin condition with itching called "atopic dermatitis", a shorter application time may be sufficient. Application times of more than 30 minutes can increase the incidence of local skin reactions (see also section 4, "Possible side effects").
- If you are being treated with products for heart rhythm disorders (class III antiarrhythmics, such as amiodarone). In this case, your doctor will monitor your heart function.
Due to the potentially greater absorption over recently shaved skin, it is essential to respect the recommended dose, skin surface, and application time.
Avoid contact of Anamap with the eyes, as it may cause irritation. If Lidocaine/Prilocaine Glenmark accidentally enters your eye, you should rinse it immediately with warm water or saline solution (sodium chloride solution). Be careful not to apply anything to the eye until sensitivity returns.
Anamap should not be applied to a damaged eardrum.
When using Anamap before being vaccinated with live vaccines (e.g., tuberculosis vaccine), revisit your doctor or nurse after the required follow-up period for the vaccination result.
Children and adolescents
In infants and newborns under 3 months, "methemoglobinemia" is frequently observed, a transient and clinically insignificant increase in blood pigment levels, up to 12 hours after Anamap application.
Clinical studies could not confirm the efficacy of Anamap when blood is drawn from the heel of newborns or to provide adequate analgesia during circumcision.
Anamap should not be applied to the genital skin (e.g., penis) or genital mucosa (e.g., vagina) of children (under 12 years) because there is insufficient data on the absorption of the active ingredients.
Anamap should not be used in children under 12 months of age who are simultaneously receiving treatment with other medications that affect the levels of the blood pigment "methemoglobin" (e.g., sulfonamides, see also section 2 "Other medications and Anamap").
Anamap should not be used in premature newborns.
Other medications and Anamap
Inform your doctor, pharmacist, or nurse if you are using, have recently used, or may need to use any other medication, including those purchased without a prescription and herbal products. This is because some medications can affect (or be affected by) the mechanism of action of Anamap.
Especially, inform your doctor, pharmacist, or nurse if you or your child have recently used any of the following medications:
- Medications used to treat infections called "sulfonamides" and nitrofurantoin.
- Medications used to treat epilepsy, called phenytoin and phenobarbital.
- Other local anesthetics.
- Medications for treating heart arrhythmias, such as amiodarone.
- Cimetidine or beta-blockers, which can increase lidocaine levels in the blood. This interaction is not clinically significant in short-term treatment with Anamap at the recommended doses.
Pregnancy, breastfeeding, and fertility
If you are pregnant, breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor, pharmacist, or nurse before using this medication.
Occasional use of Anamap during pregnancy is unlikely to have any adverse effect on the fetus.
The active ingredients of Anamap (lidocaine and prilocaine) are excreted in breast milk. However, the amount is so small that there is generally no risk to the child.
Animal studies have shown that there are no alterations in male or female fertility.
Driving and using machines
Anamap does not affect the ability to drive and use machines, or the effect is insignificant, when used at the recommended doses.
Anamap contains macrogolglycerol hydroxystearate
Macrogolglycerol hydroxystearate may cause skin reactions.
3. How to use Anamap
Follow exactly the administration instructions of this medication indicated by your doctor, pharmacist, or nurse. In case of doubt, consult your doctor, pharmacist, or nurse again.
Use of Anamap
- Where to put the cream, how much, and for how long are factors that depend on what it will be used for. Your doctor, pharmacist, or nurse will apply the cream or indicate how you should do it. Half a 5g tube corresponds to approximately 2g of Anamap. One gram of Anamap extracted from a tube is approximately 3.5 cm.
- Anamap should be applied to the genitals only by a doctor or nurse.
- When Anamap is used on leg ulcers, the application should be supervised by a doctor or nurse.
Do not use Anamap on the following areas:
- Cuts, scratches, or wounds, except for leg ulcers.
- Areas with eczema or irritation.
- Eyes or near them.
- Inside the nose, ear, or mouth.
- Anal orifice (anus).
- Genital mucosa of children.
People who frequently apply or remove the cream should ensure they avoid contact to prevent the appearance of hypersensitivity.
The protective membrane of the tube is pierced by pressing the cap onto it.
Use on the skinbefore small interventions (such as needle puncture or minor skin surgery):
- Apply a thick layer of cream to the skin. Follow the instructions in the leaflet or those of a healthcare professional who will indicate where to apply it. In some cases, the healthcare professional must apply the cream.
- Cover the cream with a dressing [plastic wrap] afterwards. This is removed just before starting the intervention. If you apply the cream yourself, make sure your doctor, pharmacist, or nurse provides you with the dressings.
- The usual dose for adults and adolescents over 12 years is 2g (grams).
- In adults and adolescents over 12 years, apply the cream at least 60 minutes before the intervention (unless the cream is to be used on the genitals). However, do not apply it more than 5 hours before.
- For children, the amount of Anamap used and how long it is applied depends on their age. Your doctor, pharmacist, or nurse will tell you how much to use and when it should be applied.
It is very important that you follow the following instructions when applying the cream:
- Squeeze the tube to apply the necessary amount of cream to the skin where the intervention will be performed (e.g., where the needle will be inserted). Half a 5g tube corresponds to approximately 2g of Anamap. One gram of Anamap extracted from the tube is approximately 3.5 cm.
Do not spread the cream.
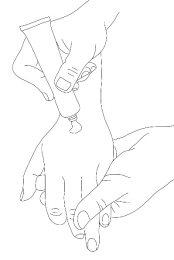
- Peel off the paper layer of the occlusive dressing.
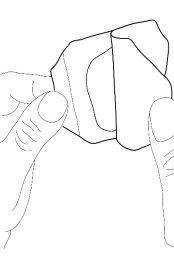
- Remove the covers of the occlusive dressing and then carefully place it over the cream mound. Do not spread the cream covered by the dressing.
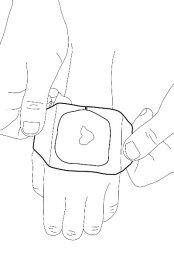
- Remove the plastic layer. Smooth the edges of the occlusive dressing carefully. Then leave it on for at least 60 minutes if the skin is not damaged. The cream should not be left on for more than 60 minutes in children under 3 months or more than 30 minutes in children with a skin condition that causes itching called "atopic dermatitis". If the cream is used on the genitals or over ulcers, the application times may be shorter as described below.
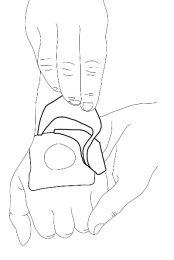
- The doctor or nurse will remove the occlusive dressing and clean the cream before starting the intervention (e.g., just before inserting the needle).
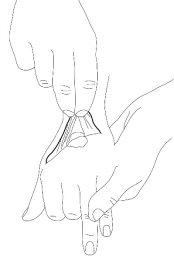
Use on larger areas of recently shaved skin before outpatient interventions (such as laser hair removal techniques)
Follow the instructions of your healthcare professional.
The recommended dose is 1g of cream per 10cm2 (10 square centimeters) of extension, applied 1 to 5 hours under an occlusive dressing. Anamap should not be used on recently shaved areas of more than 600cm2 (600 square centimeters, e.g., 30cm x 20cm) of extension. The maximum dose is 60g.
Before hospital interventions (such as skin grafts) that require deeper anesthesia
- Anamap can be used in this way in adults and adolescents over 12 years, but only under the supervision of a doctor or nurse.
- The recommended dose is 1.5g to 2g of cream per 10cm2 (10 square centimeters) of extension.
- After applying the cream, it should be covered with an occlusive dressing for a minimum of 2 hours and a maximum of 5 hours.
Use on the skin to remove verrucous lesions called "molluscum"
- Anamap can be used in children and adolescents who have a skin condition called "atopic dermatitis".
- The usual dose depends on the child's age and is used for 30 to 60 minutes (30 minutes if the patient has atopic dermatitis). Your doctor, nurse, or pharmacist will indicate how much cream to apply.
Use on the genital skin before local anesthesia injection
- Anamap can be used in this way only in adults and adolescents over 12 years and only applied by a healthcare professional.
- The recommended dose is 1g of cream (1g to 2g for female genital skin) per 10cm2 (10 square centimeters) of extension.
- The cream is applied under an occlusive dressing. This is maintained for 15 minutes on male genital skin and for 60 minutes on female genital skin.
Use on the genitals before minor skin surgery (such as wart removal)
Anamap can be used in this way only in adults and adolescents over 12 years and only applied by a healthcare professional.
The recommended dose is 5g to 10g of cream for 10 minutes. Without an occlusive dressing. The medical intervention should start immediately.
Use before cleaning or debridement of leg ulcers
- Anamap can be used in this way in adults, but only under the supervision of a doctor or nurse.
- The recommended dose is 1g to 2g of cream per 10cm2 up to a maximum of 10g.
- The cream is applied under an occlusive dressing, for example, a plastic wrap. This is maintained for 30 to 60 minutes before cleaning the ulcer. Remove the cream with a cotton swab and start cleaning without delay.
- Anamap can be used before cleaning leg ulcers up to a maximum of 15 times during a period of 1 to 2 months.
- The Anamap tube is for single use when used on leg ulcers: The tube with any remaining content should be discarded each time after treating a patient.
If you use more Anamap than indicated:
If you use more Anamap than described in this leaflet or more than indicated by your doctor, pharmacist, or nurse, contact them immediately, even if you do not have any symptoms.
The symptoms produced by using too much Anamap are as follows. The appearance of these symptoms is very unlikely if Anamap is used as recommended.
- Dizziness or lightheadedness.
- Tingling of the skin around the mouth and numbness of the tongue.
- Abnormal taste.
- Blurred vision.
- Ringing in the ears.
- There is also a risk of acute methemoglobinemia (a problem with blood pigmentation levels). This is more frequent when taking certain medications. In these cases, the skin turns a bluish-gray color due to lack of oxygen.
In severe cases of overdose, symptoms can include seizures, low blood pressure, slow breathing, cessation of breathing, and alterations in pulse. These effects can be potentially fatal.
If you have any questions about the use of this medication, consult your doctor, pharmacist, or nurse.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Contact your doctor or pharmacist if any of the following adverse effects cause you discomfort or do not seem to disappear. Inform your doctor of anything else that makes you feel unwell while using Anamap.
A mild reaction (pallor or redness of the skin, slight swelling, initial burning or itching) may appear in the area where Anamap is applied. These are normal reactions to the cream and anesthetics and will disappear shortly without the need for any action.
If you experience any unpleasant or unusual effect while using Anamap, stop using it and consult your doctor or pharmacist as soon as possible.
Frequent(may affect 1 in 10 people):
- Local transient skin reactions (pallor, redness, swelling) in the application area during treatment on the skin, genital mucosa, or leg ulcers
- A mild initial sensation of burning, itching, or heat in the application area during treatment on the genital mucosa or leg ulcers.
Uncommon(may affect 1 in 100 people):
- A mild initial sensation of burning, itching, or heat in the treated area during treatment on the skin.
- Numbness (tingling) in the application area during treatment on the genital mucosa.
- Skin irritation in the application area during treatment of leg ulcers.
Rare(may affect 1 in 1000 people):
- Allergic reactions that, in rare cases, can lead to anaphylactic shock (skin rash, swelling, fever, difficulty breathing, and fainting) during treatment on the skin, genital mucosa, or leg ulcers.
- Methemoglobinemia (blood disorder) during treatment of the skin.
- Small pinpoint bleeding in the treated area (particularly in children with eczema after long periods of application) during treatment of the skin.
- Eye irritation if Anamap accidentally comes into contact with the eyes during treatment of the skin.
Other Adverse Effects in Children
Methemoglobinemia, a blood disorder that is usually observed more frequently in newborns and infants from 0 to 12 months, often associated with overdose.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that is not listed in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Conservation of Anamap
Store at less than 30°C, do not refrigerate or freeze.
Use within 6 months of first opening.
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date indicated on the packaging after CAD. The expiration date is the last day of the month indicated.
Medicines should not be thrown away through wastewater or household waste. Deposit the packaging and medicines you no longer need at the SIGRE point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Anamap
- The active ingredients are lidocaine and prilocaine. Each gram of cream contains 25 mg of lidocaine and 25 mg of prilocaine.
- The other components are hydroxystearate of macrogolglycerol (hydrogenated castor oil polyoxyl), carbomer (974P), sodium hydroxide, and purified water.
Appearance of the Product and Package Contents
Anamap is a soft white cream. It is presented in a 5 g and 30 g collapsible aluminum tube, internally coated with an epoxypenolic lacquer.
Package sizes:
1 x 30 g tube
1 x 5 g tube
1 x 5 g tube with 2 dressings
1 x 5 g tube with 3 dressings
5 x 5 g tubes
5 x 5 g tubes with 12 dressings
Not all package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing authorization holder:
Glenmark Arzneimittel GmbH
Industriestr. 31
82194 Gröbenzell
Germany
Manufacturer:
Rafarm SA
Thesi Pousi-Xatzi
Agiou Louka
Paiania, Attiki-19002
P.P. Box 37
Greece
Qualimetrix SA
579 Mesogeion avenue, Agia Paraskevi
Athens, 15343
Greece
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Glenmark Farmacéutica, S.L.U.
C/ Retama 7, 7th floor
28045 Madrid
Spain
This medicine is authorized in the member states of the European Economic Area under the following names:
Denmark: Nulbia
Bulgaria: ROMLA 25mg/25mg/g
Germany: Emulus® 25mg/g + 25mg/g Cream
Poland: MOTTI
Romania: ROMLA 25mg/25mg/g Cream
Sweden: Lidokain/Prilokain Alternova
United Kingdom: Nulbia 5% Cream
Spain: Anamap 25mg/g + 25mg/g cream
Czech Republic: ROMLA
Slovakia: ROMLA
Date of the last revision of this prospectus:April 2021
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Average pharmacy price10.02 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ANAMAP 25 mg/g + 25 mg/g CREAMDosage form: CREAM, 25 mg/g + 25 mg/gActive substance: combinationsManufacturer: Galenicum Derma S.L.U.Prescription requiredDosage form: CREAM, Lidocaine 25 mg/g + Prilocaine 25 mg/gActive substance: combinationsManufacturer: Mesoestetic Pharma Group S.L.Prescription requiredDosage form: CREAM, 25 mg lidocaine; 25 mg prilocaine/ gActive substance: combinationsManufacturer: Aspen Pharma Trading LimitedPrescription required
Online doctors for ANAMAP 25 mg/g + 25 mg/g CREAM
Discuss questions about ANAMAP 25 mg/g + 25 mg/g CREAM, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















