AMVUTTRA 25 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA

Cómo usar AMVUTTRA 25 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Amvuttra 25mg solución inyectable en jeringa precargada
vutrisirán
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Amvuttra y para qué se utiliza
- Qué necesita saber antes de usar Amvuttra
- Cómo usar Amvuttra
- Posibles efectos adversos
- Conservación de Amvuttra
- Contenido del envase e información adicional
1. Qué es Amvuttra y para qué se utiliza
El principio activo de Amvuttra es vutrisirán.
Para qué se utiliza Amvuttra
Amvuttra se utiliza para el tratamiento de una enfermedad llamada “amiloidosis ATTR”. Esta enfermedad puede ser hereditaria y también puede deberse al envejecimiento. La amiloidosis ATTR está causada por problemas de una proteína del organismo llamada “transtiretina” (TTR). Esta proteína se forma en su mayor parte en el hígado y transporta la vitamina A y otras sustancias por el organismo.
En las personas con esta enfermedad, unas pequeñas fibras de la proteína TTR se agrupan para formar depósitos llamados “amiloide”. El amiloide se puede acumular en torno a los nervios, el corazón y otros lugares del cuerpo o en el interior de estos e impedir que funcionen con normalidad. Esto provoca los síntomas de la enfermedad.
Cómo actúa Amvuttra
Amvuttra actúa reduciendo la cantidad de proteína TTR que produce el hígado, lo que significa que hay menos proteína TTR en la sangre que pueda formar amiloide. Esto puede ayudar a reducir los efectos de esta enfermedad.
Amvuttra solo se utiliza en adultos.
2. Qué necesita saber antes de usar Amvuttra
No use Amvuttra
- si ha sufrido alguna vez una reacción alérgica grave a vutrisirán o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Si no está seguro, consulte a su médico, farmacéutico o enfermero antes de usar este medicamento.
Advertencias y precauciones
Niveles reducidos de vitaminaA en la sangre y suplementos de vitamina
Amvuttra reduce la cantidad de vitamina A de la sangre.
Su médico le pedirá que tome un suplemento de vitamina A diario. Tome la dosis de vitamina A recomendada por su médico.
Los signos de niveles bajos de vitamina A pueden incluir: problemas de la visión, especialmente por la noche, ojos secos o visión borrosa.
- Si nota algún cambio en su visión o cualquier otro problema ocular mientras usa Amvuttra, consulte a su médico. Su médico puede derivarle a un oculista para una revisión.
Unos niveles demasiado altos o demasiado bajos de vitamina A pueden perjudicar el desarrollo del feto. Por lo tanto, se debe excluir el embarazo en las mujeres en edad fértil antes de comenzar el tratamiento con Amvuttra y tienen que utilizar métodos anticonceptivos efectivos (ver sección “Embarazo, lactancia y anticoncepción” a continuación).
- Los niveles de vitamina A pueden permanecer bajos durante más de 12 meses después de la última dosis de Amvuttra.
- Informe a su médico si tiene previsto quedarse embarazada. Su médico le indicará que deje de usar Amvuttra y el suplemento de vitamina A. Su médico también se asegurará de que sus niveles de vitamina A hayan vuelto a la normalidad antes de intentar quedarse embarazada.
- Informe a su médico en caso de un embarazo no planificado. Su médico le indicará que deje de usar Amvuttra. Durante los primeros 3 meses del embarazo, su médico puede indicarle que deje de tomar el suplemento de vitamina A. Durante los últimos 6 meses de embarazo, su médico puede indicarle que reanude el suplemento de vitamina A si los niveles de vitamina A en su sangre aún no han vuelto a la normalidad, debido a un mayor riesgo de deficiencia de esta vitamina durante los últimos 3 meses de embarazo.
Niños y adolescentes
Amvuttra no está recomendado en niños y adolescentes de menos de 18 años de edad.
Otros medicamentos y Amvuttra
Informe a su médico, farmacéutico o enfermero si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Embarazo, lactancia y anticoncepción
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Embarazo
No use Amvuttra si está embarazada.
Mujeres en edad fértil
Amvuttra reducirá el nivel de vitamina A en su sangre, la cual es importante para el desarrollo normal del feto (ver “Advertencias y precauciones” más arriba).
- Si es usted una mujer que puede quedarse embarazada, debe usar un método anticonceptivo efectivo durante el tratamiento con Amvuttra.
- Consulte a su médico o enfermero sobre los métodos anticonceptivos adecuados.
- Se debe excluir el embarazo antes de comenzar el tratamiento con Amvuttra.
- Informe a su médico si tiene intención de quedarse embarazada o en caso de un embarazo no planificado. Su médico le indicará que deje de tomar Amvuttra.
Lactancia
Se desconoce si vutrisirán puede pasar a la leche materna. Su médico sopesará los posibles beneficios del tratamiento para usted frente a los riesgos de la lactancia para el bebé.
Conducción y uso de máquinas
Se cree que la influencia de Amvuttra sobre la capacidad para conducir o usar máquinas es nula o insignificante. Su médico le dirá si su afección le permite conducir vehículos y utilizar máquinas con seguridad.
Amvuttra contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por ml; esto es, esencialmente “exento de sodio”.
3. Cómo usar Amvuttra
Amvuttra puede ser autoadministrado o administrado por un cuidador o por un profesional sanitario.
Su médico o profesional sanitario le mostrará a usted y/o a su cuidador cómo preparar e inyectar una dosis de Amvuttra antes de que lo haga usted mismo..
Para saber cómo debe usar Amvuttra, lea las “Instrucciones de uso” al final de este prospecto.
Cuánto Amvuttra debe usar
La dosis recomendada es de 25 mg una vez cada 3 meses.
Dónde se administra la inyección
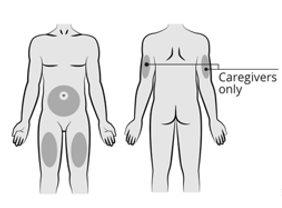
Amvuttra se administra mediante inyección bajo la piel (“inyección subcutánea”) en la zona del estómago (abdomen), en la parte superior del brazo (si otra persona le administra la inyección) o en el muslo.
Cuánto tiempo debe usar Amvuttra
Su médico le dirá cuánto tiempo necesita usar Amvuttra. No interrumpa el tratamiento con Amvuttra a menos que su médico se lo indique.
Si usa más Amvuttra del que debe
En el caso improbable de que use demasiado (una sobredosis), póngase en contacto con su médico o farmacéutico, incluso si no presenta síntomas. Su médico comprobará si tiene efectos adversos.
Si olvidó usar Amvuttra
Si se olvida una dosis, administre Amvuttra lo antes posible. A partir de entonces, reanude la administración cada 3 meses, contando desde la dosis administrada más recientemente.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Informe a su médico, farmacéutico o enfermero si nota alguno de los siguientes efectos adversos:
Frecuentes:pueden afectar hasta 1 de cada 10 personas
- Enrojecimiento, dolor, picor, cardenales o calor en el lugar de inyección
- Análisis de sangre que muestran aumentos en las enzimas hepáticas llamadas fosfatasa alcalina y alanina transaminasa
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Amvuttra
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta, en la tapa de la bandeja y en la caja después de CAD/EXP. La fecha de caducidad es el último día del mes que se indica.
No conservar a temperatura superior a 30 °C.
No congelar.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Amvuttra
- El principio activo es vutrisirán.
Cada jeringa precargada contiene vutrisirán sódico equivalente a 25 mg de vutrisirán en 0,5 ml de solución.
- Los demás componentes son: dihidrogenofosfato de sodio dihidrato, fosfato disódico dihidrato, cloruro de sodio y agua para preparaciones inyectables. Se puede usar hidróxido de sodio y ácido fosfórico para ajustar el pH (consulte “Amvuttra contiene sodio” en la sección 2).
Aspecto del producto y contenido del envase
Este medicamento es una solución inyectable (inyección) transparente, de incolora a amarilla. Cada envase contiene una jeringa precargada para un solo uso.
Titular de la autorización de comercialización y responsable de la fabricación
Alnylam Netherlands B.V.
Antonio Vivaldistraat 150
1083 HP Ámsterdam
Países Bajos
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Alnylam Netherlands B.V. Tél/Tel: 0800 81 443 (+32 234 208 71) | Luxembourg/Luxemburg Alnylam Netherlands B.V. Tél/Tel: 80085235 (+352 203 014 48) |
Ceská republika Medison Pharma s.r.o. Tel: + 31 20 369 7861 Danmark Alnylam Sweden AB Tlf: 433 105 15 (+45 787 453 01) | Lietuva Medison Pharma Lithuania UAB Tel: +31 20 369 7861 Magyarország Medison Pharma Hungary Kft Tel.: +31 20 369 7861 Malta Genesis Pharma (Cyprus) Ltd Tel: +357 22765715 |
Deutschland Alnylam Germany GmbH Tel: 08002569526 (+49 8920190112) | Nederland Alnylam Netherlands B.V. Tel: 0800 282 0025 (+31 20 369 7861) |
Eesti Medison Pharma Estonia OÜ Tel: +31 20 369 7861 | Norge Alnylam Sweden AB Tlf: 800 544 00 (+472 1405 657) |
Ελλ?δα ΓΕΝΕΣΙΣ ΦΑΡΜΑ Α.Ε Τηλ: +30 210 87 71 500 España Alnylam Pharmaceuticals Spain SL Tel: 900810212 (+34 910603753) | Österreich Alnylam Austria GmbH Tel: 0800070339 (+43 720 778 072) Polska Medison Pharma Sp. z o.o. Tel: +31 20 369 7861 |
France Alnylam France SAS Tél: 0805542656 (+33 187650921) | Portugal Alnylam Portugal Tel: 707201512 (+351 21 269 853) |
Hrvatska Genesis Pharma Adriatic d.o.o Tel: +385 1 5530 011 | România Genesis Biopharma Romania SRL Tel: +40 21 403 4074 |
Ireland Alnylam Netherlands B.V. Tel: 1800 924260 (+353 818 882213) Ísland Alnylam Netherlands B.V. Sími: +31 20 369 7861 | Slovenija Genesis Biopharma SL d.o.o Tel: +386 1 292 70 90 Slovenská republika Medison Pharma s.r.o. Tel: +31 20 369 7861 |
Italia Alnylam Italy S.r.l. Tel: 800 90 25 37 (+39 02 89 73 22 91) | Suomi/Finland Alnylam Sweden AB Puh/Tel: 0800 417 452 (+358 942 727 020) |
Κ?προς Genesis Pharma (Cyprus) Ltd Τηλ: +357 22765715 | Sverige Alnylam Sweden AB Tel: 020109162 (+46 842002641) |
Latvija Medison Pharma Latvia SIA Tel: +31 20 369 7861 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu/.
-------------------------------------------------------------------------------------------------------------------
INSTRUCCIONES DE USO
Amvuttra 25mg solución inyectable en jeringa precargada
vutrisirán
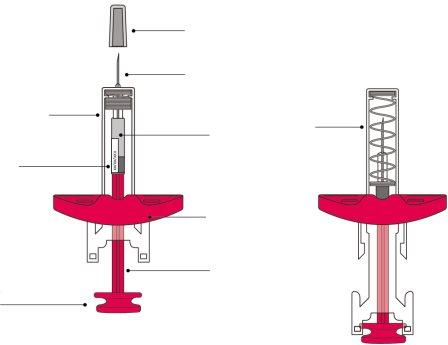
Jeringa precargada unidosis con protector de aguja
Lea estas instrucciones antes de utilizar esta jeringa precargada.
Información sobre la jeringa precargada
La jeringa precargada (denominada “jeringa”) es desechable y de un solo uso.
Vía y forma de administración
Cada caja contiene una jeringa de un solo uso de Amvuttra. Cada jeringa de Amvuttra contiene 25 mg de vutrisirán para inyectar bajo la piel (inyección subcutánea), una vez cada 3 meses.
Su médico o profesional sanitario le mostrará a usted y/o a su cuidador cómo preparar e inyectar una dosis de Amvuttra antes de que lo haga usted mismo. Póngase en contacto con su profesional sanitario o su médico para obtener más información y ayuda en caso necesario.
Conserve estas instrucciones hasta que haya utilizado la jeringa.
Conservación de Amvuttra
Noconservar a temperatura superior a 30 °C.
Nocongelar.
Mantener este medicamento fuera de la vista y del alcance de los niños.
Advertencias importantes
Noutilice el medicamento si la caja está dañada o presenta signos de manipulación.
Noutilice la jeringa si se ha caído sobre una superficie dura.
Notoque el vástago del émbolo hasta que esté listo para inyectar.
Noretire la cápsula de cierre de la aguja hasta justo antes de la inyección.
Novuelva a colocar la cápsula de cierre en la jeringa en ningún momento.
Aspecto de la jeringa antes y después de usarla: |
Antes de usarlaDespués de usarla
|
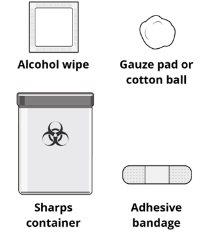
Paso1: Reúna los materiales Reúna y coloque los siguientes materiales (no suministrados) sobre una superficie plana y limpia:
|
|
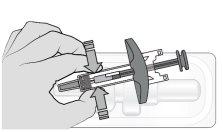
Paso2: Prepare la jeringa Si se conserva en nevera, deje la jeringa a temperatura ambiente durante 30 minutos para que se atempere antes de usarla. Nocaliente la jeringa de ninguna otra forma, p. ej., microondas, agua caliente o cerca de otras fuentes de calor. Saque la jeringa del envase sujetándola por el cilindro. Notoque el vástago del émbolo hasta que esté listo para administrar la inyección. Noutilice la jeringa si se ha caído sobre una superficie dura. Noretire la cápsula de cierre de la aguja hasta justo antes de la inyección. |
|
Paso3: Inspeccione la jeringa Compruebe:
Es normal ver burbujas de aire en el interior de la jeringa. Noutilice la jeringa si se detecta algún problema al comprobar la jeringa y la solución del fármaco.Nola utilice si ha superado la fecha de caducidad. Nola utilice si la solución del fármaco contiene partículas o si está turbia o presenta algún cambio de color.Póngase en contacto con su profesional sanitario si detecta algún problema. |
|
Paso4: Elija el lugar de inyección Elija un lugar de inyección de entre las zonas siguientes:
Noinyecte en zonas de la piel que estén sensibles, enrojecidas, hinchadas, con hematomas o duras o a menos de 5 cm del ombligo. |
|
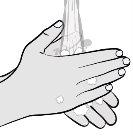
Paso5: Prepárese para la inyección Lávese las manos con agua y jabón y séqueselas bien con una toalla limpia. |
|
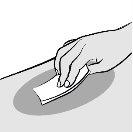
Limpie el lugar elegido para la inyección con una toallita con alcohol. Deje que la piel se seque al aire antes de la inyección. Evite tocar o soplar en el lugar de inyección después de limpiarlo. |
|
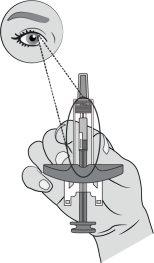
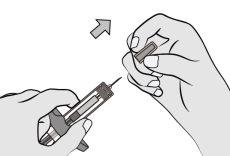
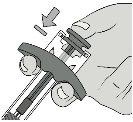
Paso6: Retire la cápsula de cierre de la agujaSujete el cilindro de la jeringa con una mano. Retire la cápsula de cierre de la aguja tirando directamente de ella con la otra mano y deséchela inmediatamente. Es normal ver una gota de líquido en la punta de la aguja. Notoque la aguja ni deje que toque ninguna superficie. Novuelva a colocar la cápsula de cierre en la jeringa. Notire del vástago del émbolo. Noutilice la jeringa si se ha caído sobre una superficie dura. |
|
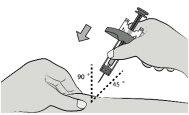
Paso7: Introduzca la aguja Con la mano libre, pellizque suavemente la piel limpia alrededor del lugar de inyección para crear una pequeña protuberancia para la inyección. |
|
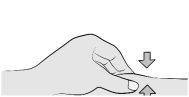
Introduzca completamente la aguja en la piel pellizcada a un ángulo de 45-90°. |
|
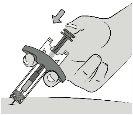
Paso8: Inyecte el medicamento Utilizando el disco de empuje, presione el vástago del émbolo mientras sujeta la jeringa por las alas de sujeción. |
|
Presione el vástago del émbolo hasta el fondo para inyectar toda la solución del fármaco. Debe presionar el vástago del émbolo hasta el fondopara administrar la dosis. |
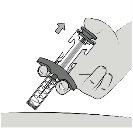
|
Paso9: Suelte el vástago del émbolo Suelte el vástago del émbolo para cubrir la aguja. Retire la jeringa de la piel. Nobloquee el movimiento del vástago del émbolo. Notire hacia abajo del protector de la aguja. El protector de la aguja cubre automáticamente la aguja. |
|
Paso10: Compruebe el lugar de inyección Puede haber una pequeña cantidad de sangre o líquido en el lugar de inyección. Si es así, presione sobre el lugar de inyección con una gasa o un algodón hasta que deje de sangrar. Evite frotar el lugar de inyección. | |
Paso11: Deseche la jeringa Deseche inmediatamentela jeringa usada en un recipiente de objetos punzocortantes. Utilice únicamente un recipiente de objetos cortopunzantespara desechar las jeringas. |
|
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a AMVUTTRA 25 MG SOLUCION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 300 mg/mlPrincipio activo: Oxibato sodioFabricante: Accord Healthcare S.L.U.Requiere recetaForma farmacéutica: COMPRIMIDO BUCODISPERSABLE/LIOTAB, 50 mgPrincipio activo: RiluzolFabricante: Zambon S.P.A.Requiere recetaForma farmacéutica: COMPRIMIDO LIBERACION MODIFICADA, 10 mgPrincipio activo: FampridinaFabricante: Accord Healthcare S.L.U.Requiere receta
Médicos online para AMVUTTRA 25 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de AMVUTTRA 25 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes