TRAVATAN 40 MICROGRAMOS/ML COLIRIO EN SOLUCION

Cómo usar TRAVATAN 40 MICROGRAMOS/ML COLIRIO EN SOLUCION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
TRAVATAN 40 microgramos/ml colirio en solución
travoprost
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es TRAVATAN y para qué se utiliza
- Qué necesita saber antes de empezar a usar TRAVATAN
- Cómo usar TRAVATAN
- Posibles efectos adversos
- Conservación de TRAVATAN
- Contenido del envase e información adicional
1. Qué es TRAVATAN y para qué se utiliza
TRAVATAN contiene travoprostque forma parte de un grupo de medicamentos llamados análogos de las prostaglandinas. Actúa disminuyendo la presión en el ojo. Se puede utilizar solo o con otras gotas oftálmicas, por ejemplo con betabloqueantes, que también reducen la presión en el ojo.
TRAVATAN se utiliza para reducir la presión elevada en el ojo en adultos, adolescentes y niños a partir de 2 meses de edad. Esta presión puede dar lugar a una enfermedad llamada glaucoma.
2. Qué necesita saber antes de empezar a usar TRAVATAN
No use TRAVATAN
- Si es alérgico al travoprost o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Consulte a su médico si se encuentra en esta situación.
Advertencias y precauciones
- TRAVATAN puede aumentarla longitud, el grosor, color y/o número de sus pestañas.También se han observado cambios como crecimiento inusual del vello en los párpados o en los tejidos de alrededor del ojo.
- TRAVATAN puede alterar el color del iris(la parte coloreada del ojo). Este cambio puede ser permanente. También puede producir un cambio de color de la piel alrededor del ojo.
- Si se ha sometido a una operación de cataratas,consulte a su médico antes de empezar a usar TRAVATAN.
- Si sufre o ha sufrido anteriormente una inflamación del ojo(iritis y uveítis), consulte a su médico antes de empezar a usar TRAVATAN.
- TRAVATAN puede, en raras ocasiones, causar falta de alientoo respiración ruidosao aumentar los síntomas del asma. Si está preocupado por los cambios en su respiración mientras utiliza TRAVATAN consulte a su médico lo antes posible.
- Travoprost puede absorberse a través de la piel. En casode contactodel medicamento con la piel,debe eliminarse lavandoinmediatamente. Esto es especialmente importante en mujeres embarazadas o que estén intentando quedarse embarazadas.
- Si lleva lentes de contacto blandas, no se aplique las gotas mientras las lleve puestas. Después de aplicar las gotas, espere 15 minutos antes de volver a colocarse las lentes.
Niños y adolescentes
Se puede usar TRAVATAN en niños desde los 2 meses de edad a menores de 18 años con la misma dosis que en adultos. No se recomienda el uso de TRAVATAN en niños menores de 2 meses de edad.
Otros medicamentos y TRAVATAN
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Embarazo, lactancia y fertilidad
No utilice TRAVATAN si está embarazada.Si cree que podría estar embarazada dígaselo en seguida a su médico. Si usted puede quedarse embarazada debe utilizar un método anticonceptivo adecuado mientras esté utilizando TRAVATAN.
No utilice TRAVATAN si está amamantando.TRAVATAN puede pasar a la leche materna.
Consulte a su médico antes de utilizar cualquier medicamento.
Conducción y uso de máquinas
Inmediatamente después de la aplicación de TRAVATAN puede notar que su visión se vuelve borrosa. No conduzca ni utilice máquinas hasta que hayan desaparecido estos efectos.
TRAVATAN contiene aceite de ricino hidrogenado y propilenglicol,que pueden causar reacciones e irritación en la piel.
3. Cómo usar TRAVATAN
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o el médico que trata al niño. En caso de duda, consulte de nuevo a su médico, al médico que trata al niño o farmacéutico.
La dosis recomendada es
Una gota en el ojo u ojos afectados, una vez al día - por la noche.
Sólo debe aplicarse TRAVATAN en los dos ojos si su médico así se lo indica. Siga el tratamiento durante todo el periodo de tiempo indicado por su médico o el médico que trata al niño.
|
|
|
|
TRAVATAN sólo debe utilizarse como gotas para sus ojos o los de los niños.
- Inmediatamente antes de utilizar un frasco por primera vez, abra la bolsa envoltorio, saque el frasco (figura 1)y anote la fecha de apertura en el espacio reservado en la caja.
- Lávese las manos.
- Desenrosque el tapón.
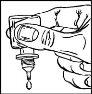
- Sostenga el frasco, boca abajo, entre los dedos.
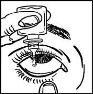
- Incline la cabeza o la cabeza del niño suavemente hacia atrás. Separe suavemente el párpado del ojo con un dedo, hasta que se forme una bolsa, en la que deberá caer la gota (figura 2).
- Acerque la punta del frasco al ojo. Puede serle útil un espejo.
- No toque el ojo, el párpado, zonas próximas ni otras superficies con el cuentagotas, porque las gotas podrían infectarse.
- Apriete suavemente el frasco para que caiga una gota de TRAVATAN cada vez (figura 3).
- Después de utilizar TRAVATAN, cierre suavemente los ojos, con suavidad presione con el dedo el borde del ojo, junto a la nariz (figura 4)durante al menos 1 minuto. Esto ayuda a evitar que TRAVATAN pase al resto del cuerpo.
- Si se aplica gotas en ambos ojos, repita los puntos anteriores para el otro ojo.
- Cierre bien el frasco inmediatamente después de utilizar el producto.
- Utilice un solo frasco a la vez. No abra la bolsa hasta que necesite utilizar el frasco.
Si una gota cae fuera del ojo, inténtelo de nuevo.
Si está usted o el niño utilizando otros medicamentos oftálmicos, como colirios o pomadas para los ojos, espere por lo menos 5 minutos entre la aplicación de TRAVATAN y de los otros medicamentos oftálmicos.
Si recibe usted o el niño más TRAVATAN del que debe
Puede eliminarlo lavando los ojos con agua templada. No se aplique más gotas hasta que le toque la siguiente dosis.
Si olvidó usar TRAVATAN
Continúe con la siguiente dosis que estaba prevista. No se aplique una dosis doblepara compensar la dosis olvidada. Nunca aplique más de una gota en el ojo(s) afectado(s) en un solo día.
Si interrumpe el tratamiento con TRAVATAN
No deje de utilizar TRAVATAN sin consultar antes con su médico o el médico que trata al niño, la presión en su ojo o del ojo del niño no estará controlada lo que podría provocar pérdida de visión.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, al médico que trata al niño o farmacéutico.
Continúa
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Puede seguir normalmente el tratamiento a no ser que los efectos adversos sean graves. Si estos efectos le preocupan, consulte a su médico o farmacéutico. No deje de aplicarse TRAVATAN sin consultar a su médico.
Con TRAVATAN se han observado los siguientes efectos adversos:
Muy frecuentes: pueden afectar a más de 1 de cada 10 personas
Efectos en el ojo:enrojecimiento del ojo.
Frecuentes: pueden afectar hasta 1 de cada 10 personas
Efectos en el ojo:cambios en el color del iris (parte coloreada del ojo), dolor en el ojo, molestia en el ojo, ojo seco, picor en el ojo, irritación en el ojo.
Poco frecuentes: pueden afectar hasta 1 de cada 100 personas
Efectos en el ojo:alteración corneal, inflamación del ojo, inflamación del iris, inflamación dentro del ojo, inflamación con o sin daño de la superficie del ojo, sensibilidad a la luz, secreción del ojo, inflamación del párpado, enrojecimiento del párpado, hinchazón alrededor del ojo, picor en el párpado, visión borrosa, aumento de la producción de lágrimas, infección o inflamación de la conjuntiva (conjuntivitis), vuelta anormal hacia fuera del párpado inferior, visión ensombrecida, costras en el párpado, crecimiento de las pestañas.
Otros efectos:aumento de los síntomas alérgicos, dolor de cabeza, frecuencia cardiaca irregular, tos, nariz taponada, irritación de garganta, oscurecimiento de la piel alrededor de los ojos, oscurecimiento de la piel, textura anormal del pelo, aumento excesivo del crecimiento del vello.
Raros: pueden afectar hasta 1 de cada 1.000 personas
Efectos en el ojo:percepción de destellos de luz, eczema de los párpados, pestañas anormalmente posicionadas que crecen hacia dentro del ojo, hinchazón del ojo, visión reducida, halo visual, sensación disminuida en el ojo, inflamación de las glándulas de los párpados, pigmentación dentro del ojo, aumento en el tamaño de la pupila, engrosamiento de las pestañas, cambio en el color de las pestañas, ojos cansados.
Otros efectos:infección vírica en el ojo, mareo, mal sabor de boca, frecuencia cardiaca irregular o disminuida, presión sanguínea aumentada o disminuida, falta de aliento, asma, inflamación o alergia nasal, sequedad nasal, cambios de voz, úlcera o malestar gastrointestinal, estreñimiento, boca seca, enrojecimiento o picor de la piel, erupción, cambio del color del vello, pérdida de pestañas, dolor articular, dolor musculo esquelético, debilidad generalizada.
Frecuencia no conocida: no puede estimarse a partir de los datos disponibles
Efectos en el ojo:inflamación de la parte posterior del ojo, ojos hundidos.
Otros efectos:depresión, ansiedad, insomnio, sensación falsa de movimiento, pitidos en oídos, dolor en el pecho, ritmo cardiaco anormal, aumento de la frecuencia cardiaca, empeoramiento del asma, diarrea, sangrados de nariz, dolor abdominal, nausea, vómitos, picor, crecimiento anormal del pelo, micción dolorosa o involuntaria (orinar de forma involuntaria o dolor al orinar), aumento del marcador del cáncer de próstata.
En niños y adolescentes, los efectos adversos más frecuentes observados con TRAVATAN son enrojecimiento del ojo y crecimiento de las pestañas. Ambos efectos adversos se observaron con una incidencia mayor en niños y adolescentes comparado con adultos.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de TRAVATAN
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice TRAVATAN después de la fecha de caducidad que aparece en el frasco y en la caja, después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere condiciones especiales de conservación.
Para evitar infecciones, debe desechar el frasco 4 semanas después de haberlo abierto por primera vez, y utilizar un frasco nuevo. Anote la fecha de apertura en el espacio reservado en la caja.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico como deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de TRAVATAN
El principio activo es travoprost 40 microgramos/ml.
Los demás componentes son: Polyquaternium-1, aceite de ricino hidrogenado y polioxietilenado 40, propilenglicol, cloruro de sodio, ácido bórico, manitol y agua purificada. Se añaden cantidades muy pequeñas de ácido clorhídrico o hidróxido de sodio para mantener los niveles de acidez (niveles de pH) normales.
Aspecto del producto y contenido del envase
TRAVATAN es un líquido (una solución incolora y transparente) que se presenta en una caja que contiene un frasco de plástico de 4 ml con un tapón de rosca. Cada frasco contiene 2,5 ml de travoprost colirio y cada frasco se encuentra dentro de una bolsa.
Tamaño de envases: 1 o 3 frascos.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Irlanda
Responsable de la fabricación
Novartis Farmacéutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona
España
Novartis Manufacturing NV
Rijksweg 14
2870 Puurs-Sint-Amands
Bélgica
Novartis Pharma GmbH
Roonstraße 25
D-90429 Nuremberg
Alemania
Siegfried El Masnou, S.A.
Camil Fabra 58
El Masnou
08320 Barcelona
España
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 | Lietuva SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
| Luxembourg/Luxemburg Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 |
Ceská republika Novartis s.r.o. Tel: +420 225 775 111 | Magyarország Novartis Hungária Kft. Tel.: +36 1 457 65 00 |
Danmark Novartis Healthcare A/S Tlf: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Deutschland Novartis Pharma GmbH Tel: +49 911 273 0 | Nederland Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Eesti SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norge Novartis Norge AS Tlf: +47 23 05 20 00 |
Ελλáδα Novartis (Hellas) A.E.B.E. Τηλ: +30 210 281 17 12 | Österreich Novartis Pharma GmbH Tel: +43 1 86 6570 |
España Novartis Farmacéutica, S.A. Tel: +34 93 306 42 00 | Polska Novartis Poland Sp. z o.o. Tel.: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tél: +33 1 55 47 66 00 | Portugal Novartis Farma - Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Hrvatska Novartis Hrvatska d.o.o. Tel. +385 1 6274 220 | România Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenija Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Novartis Slovakia s.r.o. Tel: + 421 2 5542 5439 |
Italia Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Suomi/Finland Novartis Finland Oy Puh/Tel: +358 (0)10 6133 200 |
Κúπρος Novartis Pharma Services Inc. Τηλ: +357 22 690 690 | Sverige Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvija SIA Novartis Baltics Tel: +371 67 887 070 |
Fecha de la última revisiónde este prospecto
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu
- País de registro
- Precio medio en farmacia13.35 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TRAVATAN 40 MICROGRAMOS/ML COLIRIO EN SOLUCIONForma farmacéutica: COLIRIO, 40 microgramos/mlPrincipio activo: travoprostFabricante: Alfred E. Tiefenbacher Gmbh & Co. KgRequiere recetaForma farmacéutica: COLIRIO, 40 microgramos/mlPrincipio activo: travoprostFabricante: Horus PharmaRequiere recetaForma farmacéutica: COLIRIO, 40 MICROGRAMOS/MLPrincipio activo: travoprostFabricante: Laboratorio Stada S.L.Requiere receta
Médicos online para TRAVATAN 40 MICROGRAMOS/ML COLIRIO EN SOLUCION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TRAVATAN 40 MICROGRAMOS/ML COLIRIO EN SOLUCION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes