
ТАПЕНТАДОЛ ТЕВА 100 мг ТАБЛЕТКИ ПРОЛОНГИРОВАННОГО ДЕЙСТВИЯ
Спросите врача о рецепте на ТАПЕНТАДОЛ ТЕВА 100 мг ТАБЛЕТКИ ПРОЛОНГИРОВАННОГО ДЕЙСТВИЯ

Инструкция по применению ТАПЕНТАДОЛ ТЕВА 100 мг ТАБЛЕТКИ ПРОЛОНГИРОВАННОГО ДЕЙСТВИЯ
Введение
Инструкция: информация для пациента
Тапентадол Тева 100 мг пролонгированные таблетки EFG
Прочитайте внимательно всю инструкцию перед началом приема этого препарата, поскольку она содержит важную информацию для вас.
- Сохраните эту инструкцию, поскольку вам может потребоваться перечитать ее.
- Если у вас есть какие-либо вопросы, проконсультируйтесь с вашим врачом или фармацевтом.
- Этот препарат назначен только вам и не должен быть передан другим людям, даже если они имеют такие же симптомы, как у вас, поскольку он может нанести им вред.
- Если вы испытываете побочные эффекты, проконсультируйтесь с вашим врачом или фармацевтом, даже если это побочные эффекты, которые не указаны в этой инструкции. См. раздел 4.
Содержание инструкции
- Что такое Тапентадол Тева и для чего он используется
- Что вам нужно знать перед началом приема Тапентадола Тева
- Как принимать Тапентадол Тева
- Возможные побочные эффекты
- Хранение Тапентадола Тева
- Содержание упаковки и дополнительная информация
1. Что такое Тапентадол Тева и для чего он используется
Тапентадол - активное вещество Тапентадола Тева - является сильным обезболивающим средством, принадлежащим к классу опиоидов. Тапентадол Тева используется для лечения сильной хронической боли у взрослых, которую можно адекватно лечить только опиоидными обезболивающими.
2. Что вам нужно знать перед началом приема Тапентадола Тева
Не принимайте Тапентадол Тева:
- если вы аллергичны к тапентадолу или любому другому компоненту этого препарата (указанному в разделе 6)
- если у вас астма или если ваша дыхание замедлено или слишком поверхностно (депрессия дыхания, гиперкапния)
- если у вас паралич кишечника
- если вы потребляли алкоголь, седативные препараты, другие обезболивающие или другие психотропные препараты (препараты, влияющие на настроение и эмоции) в высоких дозах (см. раздел "Другие препараты и Тапентадол Тева").
Предостережения и меры предосторожности
Проконсультируйтесь с вашим врачом или фармацевтом перед началом приема Тапентадола Тева:
- если ваше дыхание замедлено или слишком поверхностно,
- если у вас повышенное внутричерепное давление или изменение сознания до комы,
- если у вас была черепно-мозговая травма или опухоли мозга,
- если у вас есть заболевания печени или почек (см. раздел "Как принимать Тапентадол Тева"),
- если у вас есть заболевания поджелудочной железы или желчных путей, включая панкреатит,
- если вы принимаете препараты, называемые смешанными агонистами/антагонистами опиоидных рецепторов μ (например, пентазоцин, налбуфин) или частичными агонистами опиоидных рецепторов μ (например, бупренорфин),
- если вы склонны к эпилепсии или судорогам, или если вы принимаете другие препараты с известным риском увеличения судорог, поскольку риск этих приступов может увеличиться,
- если вы или члены вашей семьи имеют историю злоупотребления алкоголем, рецептурными препаратами или незаконными веществами ("аддикция"),
- если вы курите,
- если у вас когда-либо были проблемы с настроением (депрессия, тревога или расстройство личности) или если вы получали лечение от психиатра за другие психические заболевания.
Этот препарат содержит тапентадол, который является опиоидным препаратом. Повторное использование опиоидных обезболивающих может привести к привыканию к препарату (может привыкнуть к нему). Также может привести к зависимости и злоупотреблению, что может привести к потенциально смертельной передозировке. Важно сообщить вашему врачу, если вы думаете, что развили зависимость от Тапентадола Тева. Его использование (даже в терапевтических дозах) может вызвать физическую зависимость, что может привести к симптомам абстиненции и повторению ваших проблем, если вы внезапно прекратите этот фармакологический tratamiento.
Тапентадол Тева может вызывать физическую и психологическую зависимость. Если у вас есть склонность к злоупотреблению препаратами или если у вас есть зависимость от препаратов, вы должны принимать эти таблетки только в течение короткого периода времени под строгим медицинским контролем.
Расстройства дыхания, связанные со сном
Тапентадол Тева может вызывать расстройства дыхания, связанные со сном, такие как апноэ во сне (затруднение дыхания во сне) и гипоксемия, связанная со сном (пониженный уровень кислорода в крови). Симптомы могут включать паузы в дыхании во сне, ночные пробуждения из-за затруднения дыхания, трудности с поддержанием сна или чрезмерную сонливость днем. Если вы или другой человек наблюдает эти симптомы, свяжитесь с вашим врачом. Ваш врач может рассмотреть возможность снижения дозы.
Другие препараты и Тапентадол Тева
Сообщите вашему врачу или фармацевту, если вы принимаете, недавно принимали или можете принять любой другой препарат.
- Риск побочных эффектов увеличивается, если вы принимаете препараты, которые могут вызывать судороги (приступы), такие как определенные антидепрессанты или антипсихотики. Риск судорог увеличивается, если вы принимаете Тапентадол Тева одновременно с этими препаратами. Ваш врач скажет вам, подходит ли Тапентадол Тева для вас.
- Одновременное использование Тапентадола Тева и седативных препаратов, таких как бензодиазепины или связанные с ними препараты (определенные седативные препараты или транквилизаторы, например, барбитураты) или обезболивающие, такие как опиоиды, морфин и кодеин (также как препарат для кашля), антипсихотики, антигистаминные препараты H1, алкоголь, увеличивает риск сонливости, затруднения дыхания (депрессия дыхания), комы и может поставить под угрозу жизнь пациента. Из-за этого одновременное использование должно быть рассмотрено только в случае, когда нет других вариантов лечения. Однако, если ваш врач назначает вам Тапентадол Тева вместе с седативными препаратами, вам необходимо ограничить дозу и продолжительность одновременного лечения.
Одновременное использование опиоидов и препаратов, используемых для лечения эпилепсии, невропатической боли или тревоги (габапентин и прегабалин), увеличивает риск передозировки опиоидами, депрессии дыхания и может быть потенциально смертельным. Сообщите вашему врачу, если вы принимаете габапентин или прегабалин или любой другой седативный препарат, и следуйте точно рекомендации вашего врача по дозировке. Возможно, будет полезно сообщить друзьям или членам семьи, чтобы они были осведомлены о вышеуказанных симптомах. Свяжитесь с вашим врачом, когда испытываете эти симптомы.
- Если вы принимаете препарат, который влияет на уровень серотонина (например, определенные препараты для лечения депрессии), проконсультируйтесь с вашим врачом перед приемом Тапентадола Тева, поскольку были зарегистрированы случаи "серотонинового синдрома". Серотониновый синдром - это редкое, но потенциально смертельное расстройство. Симптомы могут включать непроизвольные ритмические сокращения мышц, включая мышцы, контролирующие движение глаз, агитацию, чрезмерное потоотделение, тремор, усиленные рефлексы, увеличение мышечного тонуса и повышение температуры тела выше 38°C. Ваш врач может предоставить вам дополнительную информацию.
- Не было изучено одновременное использование Тапентадола Тева с другими препаратами, называемыми смешанными агонистами/антагонистами опиоидных рецепторов μ (например, пентазоцин, налбуфин) или частичными агонистами опиоидных рецепторов μ (например, бупренорфин). Возможно, что Тапентадол Тева не будет иметь одинаковую эффективность, если его использовать одновременно с одним из этих препаратов. Сообщите вашему врачу, если вы сейчас проходите лечение одним из этих препаратов.
- Одновременное использование Тапентадола Тева и сильных ингибиторов или индукторов (например, рифампицин, фенобарбитал, зверобой) определенных ферментов, необходимых для удаления тапентадола из вашего организма, может повлиять на эффективность тапентадола или вызвать побочные эффекты, особенно при начале или прекращении этого другого препарата. Сообщите вашему врачу о всех препаратах, которые вы принимаете.
- Тапентадол Тева не должен приниматься вместе с ингибиторами МАО (препараты для лечения депрессии). Сообщите вашему врачу, если вы принимаете ингибиторы МАО или если вы принимали их в течение последних 14 дней.
Прием Тапентадола Тева с пищей, напитками и алкоголем
Не употребляйте алкоголь во время приема Тапентадола Тева, поскольку некоторые из его побочных эффектов, такие как сонливость, могут увеличиться. Прием пищи не влияет на действие этого препарата.
Беременность и лактация
Если вы беременны или кормите грудью, считаете, что можете быть беременной или планируете стать беременной, проконсультируйтесь с вашим врачом или фармацевтом перед использованием этого препарата.
Не принимайте этот препарат:
- если вы беременны, unless ваш врач назначил его вам, если используется в течение длительного периода во время беременности, тапентадол может вызвать симптомы абстиненции у новорожденного, что может поставить под угрозу жизнь новорожденного, если не будет обнаружено и behandено врачом.
- во время лактации, поскольку он может выделяться в грудное молоко.
Не рекомендуется использовать Тапентадол Тева
- во время родов, поскольку он может вызвать замедление или слишком поверхностное дыхание у новорожденного (депрессия дыхания).
Вождение и использование машин
Тапентадол Тева может вызывать сонливость, головокружение и размытое зрение и может влиять на ваши реакции. Будьте особенно осторожны при начале лечения, после изменения дозы и при одновременном использовании с алкоголем или седативными препаратами. Спросите вашего врача, можете ли вы водить машину или использовать машины во время лечения Тапентадолом Тева.
3. Как принимать Тапентадол Тева
Следуйте точно инструкциям по приему этого препарата, указанным вашим врачом или фармацевтом. В случае сомнений проконсультируйтесь с вашим врачом или фармацевтом.
Ваш врач отрегулирует дозу в зависимости от интенсивности вашей боли и вашего индивидуального уровня чувствительности к боли. Обычно необходимо принимать минимальную эффективную дозу для облегчения боли.
Взрослые
Рекомендуемая доза составляет 50 мг дважды в день, примерно каждые 12 часов.
Не рекомендуется превышать суточную дозу 500 мг тапентадола.
Ваш врач может назначить вам другую дозу или схему приема, если это необходимо. Если вы считаете, что эффект этих таблеток слишком сильный или слишком слабый, проконсультируйтесь с вашим врачом или фармацевтом.
Пациенты пожилого возраста
У пациентов пожилого возраста (старше 65 лет) обычно не требуется коррекция дозы. Однако выведение тапентадола может быть замедлено и более длительным у некоторых пациентов этой возрастной группы. Если это происходит с вами, ваш врач может назначить вам другую схему приема.
Заболевания печени и почек (печеночная и почечная недостаточность)
Пациенты с тяжелыми заболеваниями печени не должны принимать эти таблетки. Если у вас есть умеренные заболевания печени, ваш врач назначит вам другую схему приема. В случае легких заболеваний печени коррекция дозы не требуется.
Пациенты с тяжелыми заболеваниями почек не должны принимать эти таблетки. В случае легких или умеренных заболеваний почек коррекция дозы не требуется.
Использование у детей и подростков
Тапентадол Тева не показан для использования у детей и подростков моложе 18 лет.
Как и когда принимать Тапентадол Тева
Тапентадол Тева должен приниматься перорально.
Всегда проглатывайте таблетки целиком с достаточным количеством жидкости.
Не жуйте и не дробите их, поскольку это может привести к передозировке, поскольку активное вещество будет высвобождено в вашем организме слишком быстро.
Вы можете принимать их натощак (на пустой желудок) или с пищей.
Таблетку можно разделить на равные дозы.
Покрытие таблетки может не быть полностью переварено и, следовательно, появиться в кале. Это не должно беспокоить вас, поскольку активное вещество таблетки уже будет абсорбировано организмом, и то, что вы видите, - это только пустое покрытие.
Инструкции по открытию блистера
Этот препарат упакован в блистер-пакет с защитой от детей. Не можно выдавить таблетку через блистер. Обратите внимание на следующие инструкции по открытию блистера:
- Разрежьте одну дозу вдоль линий разреза на блистере.
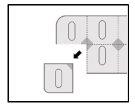
- Найдите незапечатанную зону, расположенную там, где линии разреза пересекаются.
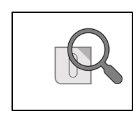
- Потяните за незапечатанную зону, чтобы оторвать ламинат
Как долго вы должны принимать Тапентадол Тева
Не принимайте таблетки дольше, чем указано вашим врачом.
Если вы приняли слишком много Тапентадола Тева
После приема очень высоких доз вы можете испытать следующие эффекты:
- сильно суженные зрачки,
- рвота,
- снижение артериального давления,
- учащенное сердцебиение,
- обморок, изменение сознания или кома (глубокая потеря сознания),
- судороги,
- замедление или слишком поверхностное дыхание до опасного уровня или остановка дыхания.
Если вы испытываете любой из этих эффектов, немедленно позвоните врачу.
В случае передозировки или случайного приема проконсультируйтесь с вашим врачом или фармацевтом или позвоните в Центр токсикологической информации, телефон: 91 562 04 20, указав препарат и количество, использованное. Рекомендуется взять с собой упаковку и инструкцию к препарату к медицинскому специалисту.
Если вы забыли принять Тапентадол Тева
Если вы забыли принять таблетку, вероятно, что боль вернется. Не принимайте двойную дозу, чтобы компенсировать пропущенные дозы, а продолжайте принимать таблетки, как раньше.
Если вы прекратите лечение Тапентадолом Тева
Если вы прекратите или остановите лечение слишком рано, вероятно, что боль вернется. Если вы хотите прекратить лечение, проконсультируйтесь с вашим врачом перед этим.
Обычно пациенты не испытывают побочных эффектов после прекращения лечения, но в редких случаях люди, принимавшие таблетки в течение длительного времени, могут чувствовать себя плохо, если внезапно прекратят принимать их.
Симптомы могут включать:
- беспокойство, слезотечение, насморк, зевание, потоотделение, озноб, мышечную боль и расширение зрачков,
- раздражительность, тревогу, боль в спине, суставную боль, слабость, мышечные спазмы, трудности с засыпанием, тошноту, потерю аппетита, рвоту, диарею и повышение артериального давления, частоты дыхания или сердечного ритма.
Если вы испытываете любой из этих симптомов после прекращения лечения, проконсультируйтесь с вашим врачом.
Не прекращайте внезапно этот препарат, если только ваш врач не скажет вам об этом. Если ваш врач хочет, чтобы вы прекратили принимать эти таблетки, он укажет вам, как это сделать, что может включать постепенное снижение дозы.
Если у вас есть какие-либо другие вопросы о использовании этого препарата, проконсультируйтесь с вашим врачом или фармацевтом.
4. Возможные побочные эффекты
Как и все лекарства, это лекарство может вызывать побочные эффекты, хотя не все люди испытывают их.
Побочные эффекты или симптомы, на которые следует обратить внимание и что делать, если вы испытываете их:
- Это лекарство может вызывать аллергические реакции. Симптомы могут включать свистящее дыхание (вид свиста при дыхании), затруднение дыхания, отек век, лица или губ, кожную сыпь или зуд, особенно те, которые поражают все тело.
- Другой серьезный побочный эффект заключается в более медленном или слабом дыхании, чем обычно. Это происходит в основном у пожилых пациентов или у ослабленных пациентов.
Если вы испытываете любой из этих важных симптомов, обратитесь к врачу немедленно.
Другие побочные эффекты, которые могут возникнуть:
Очень частые(могут возникнуть у более 1 из 10 человек)
- тошнота, запор,
- головокружение, сонливость, головная боль.
Частые(могут возникнуть у до 1 из 10 человек)
- уменьшение аппетита, тревога, депрессия настроения, трудности со сном, нервозность, беспокойство, нарушения внимания,
- дрожь, мышечные тики,
- приливы,
- одышка,
- рвота, диарея, плохая digestия,
- зуд, увеличение потоотделения, кожные высыпания,
- чувство слабости, усталость, чувство изменения температуры тела, сухость слизистых, накопление воды в тканях (отек).
Редкие(могут возникнуть у до 1 из 100 человек)
- аллергическая реакция на лекарства (включая отек под кожей, крапивницу и в тяжелых случаях затруднение дыхания, снижение артериального давления, коллапс или шок),
- потеря веса,
- дезориентация, спутанность сознания, возбуждение (агитация), нарушения восприятия, нарушения сна, эйфорическое настроение, депрессия уровня сознания, ухудшение памяти, умственное ухудшение,
- обморок, седация, нарушения равновесия, трудности с речью, онемение, аномальные ощущения в коже (например, покалывание, зуд),
- нарушения зрения,
- ускоренное сердцебиение, замедленное сердцебиение, сердечные перебои, снижение артериального давления,
- боль в животе,
- сыпь,
- задержка мочи, частое мочеиспускание,
- сексуальная дисфункция,
- синдром отмены лекарства (см. раздел "Если вы прекратите лечение Тапентадолом Тева"), чувство дискомфорта, раздражительность.
Очень редкие(могут возникнуть у до 1 из 1000 человек)
- зависимость от лекарства, нарушение мышления, эпилептические приступы, чувство предобморочного состояния, нарушение координации,
- медленное или слишком поверхностное дыхание до опасных уровней (угнетение дыхания),
- нарушение опорожнения желудка,
- чувство опьянения, чувство расслабления.
Частота неизвестна(частота не может быть оценена на основе доступных данных)
- делирий.
В целом, вероятность мыслей и поведения, связанного с суицидом, увеличивается у пациентов с хроническими болями. Кроме того, некоторые лекарства для лечения депрессии (влияющие на систему нейротрансмиттеров мозга) могут увеличить этот риск, особенно в начале лечения. Хотя тапентадол также влияет на нейротрансмиттеры, через опыт пациентов не было доказано, что он увеличивает этот риск.
Сообщение о побочных эффектах
Если вы испытываете любой побочный эффект, обратитесь к врачу или фармацевту, даже если это возможные побочные эффекты, которые не указаны в этом листке. Вы также можете сообщить о них直接 через Систему испанской фармаковигиланции лекарств для человека: https://www.notificaram.es. Сообщая о побочных эффектах, вы можете способствовать предоставлению более полной информации о безопасности этого лекарства.
5. Хранение Тапентадола Тева
Храните это лекарство вне поля зрения и досягаемости детей.
Не используйте это лекарство после даты истечения срока годности, указанной на упаковке и на блистере после CAD. Дата истечения срока годности - последний день месяца, указанного.
Это лекарство не требует особых условий хранения.
Лекарства не следует выбрасывать в канализацию или мусор. Поместите упаковку и лекарства, которые вам больше не нужны, в Пункт SIGRE аптеки. В случае сомнений спросите у фармацевта, как избавиться от упаковки и лекарств, которые вам больше не нужны. Таким образом, вы поможете защитить окружающую среду.
6. Содержание упаковки и дополнительная информация
Состав Тапентадола Тева
- Активное вещество - тапентадол.
Тапентадол Тева 100 мг пролонгированные таблетки EFG
Каждая таблетка содержит тапентадол фосфат, эквивалентный 100 мг тапентадола.
- Другие компоненты:
Ядро таблетки: микрокристаллическая целлюлоза (E460), гипромеллоза (E464), коллоидный алюмосиликат (E551), стеарат магния.
Покрытие таблетки:гипромеллоза (E464), глицерол (E422), тальк (E553b), микрокристаллическая целлюлоза (E460), диоксид титана (E171), оксид железа красный (E172), оксид железа желтый (E172), оксид железа черный (E172).
Внешний вид продукта и содержание упаковки
Тапентадол Тева 100 мг - пролонгированные таблетки желтого цвета, овальной формы, двояковыпуклые (7 мм x 14 мм) с насечками на обеих сторонах.
Таблетку можно разделить на две равные части.
Тапентадол Тева 100 мг пролонгированные таблетки выпускаются в следующих размерах упаковки:
20x1, 24x1, 30x1, 50x1, 54x1, 60x1 или 100x1 пролонгированные таблетки в блистерах-упаковках с защитой от детей.
Возможно, не все размеры упаковки будут выпускаться.
Владелец разрешения на маркетинг и ответственное лицо за производство
Владелец разрешения на маркетинг
Teva Pharma, S.L.U.
К/ Анабель Сегура 11, Эдифисио Альбатрос Б, 1-я плоскость.
28108 Алькобендас. Мадрид (Испания).
Ответственное лицо за производство
Develco Pharma GmbH
Гринматт 27
79650 Шопфхайм
Германия
Дата последнего обзора этого листка: сентябрь 2022
Подробная и актуальная информация о этом лекарстве доступна на сайте Испанского агентства по лекарствам и медицинским продуктам (AEMPS) http://www.aemps.gob.es

Сколько стоит ТАПЕНТАДОЛ ТЕВА 100 мг ТАБЛЕТКИ ПРОЛОНГИРОВАННОГО ДЕЙСТВИЯ в Испании в 2025 году?
Средняя цена на ТАПЕНТАДОЛ ТЕВА 100 мг ТАБЛЕТКИ ПРОЛОНГИРОВАННОГО ДЕЙСТВИЯ в декабрь, 2025 года составляет около 44.02 евро. Финальная стоимость может зависеть от региона, конкретной аптеки и рецептурного статуса. Для точной информации лучше проверить онлайн или в ближайшей аптеке.
- Страна регистрации
- Средняя цена в аптеках44.02 EUR
- Активное вещество
- Требуется рецептДа
- Производитель
- Информация носит справочный характер и не является медицинской рекомендацией. Перед приемом любых препаратов проконсультируйтесь с врачом. Oladoctor не несет ответственности за медицинские решения, принятые на основе этого контента.
- Аналоги ТАПЕНТАДОЛ ТЕВА 100 мг ТАБЛЕТКИ ПРОЛОНГИРОВАННОГО ДЕЙСТВИЯФорма выпуска: ТАБЛЕТКА С МОДИФИЦИРОВАННЫМ ВЫСВОБОЖДЕНИЕМ, 100 мгАктивное вещество: tapentadolПроизводитель: Sandoz Farmaceutica S.A.Требуется рецептФорма выпуска: ТАБЛЕТКА С МОДИФИЦИРОВАННЫМ ВЫСВОБОЖДЕНИЕМ, 150 мгАктивное вещество: tapentadolПроизводитель: Sandoz Farmaceutica S.A.Требуется рецептФорма выпуска: ТАБЛЕТКА С МОДИФИЦИРОВАННЫМ ВЫСВОБОЖДЕНИЕМ, 200 мгАктивное вещество: tapentadolПроизводитель: Sandoz Farmaceutica S.A.Требуется рецепт
Аналоги ТАПЕНТАДОЛ ТЕВА 100 мг ТАБЛЕТКИ ПРОЛОНГИРОВАННОГО ДЕЙСТВИЯ в других странах
Лучшие аналоги с тем же действующим веществом и терапевтическим эффектом.
Аналог ТАПЕНТАДОЛ ТЕВА 100 мг ТАБЛЕТКИ ПРОЛОНГИРОВАННОГО ДЕЙСТВИЯ в Polska
Аналог ТАПЕНТАДОЛ ТЕВА 100 мг ТАБЛЕТКИ ПРОЛОНГИРОВАННОГО ДЕЙСТВИЯ в Ukraina
Врачи онлайн по ТАПЕНТАДОЛ ТЕВА 100 мг ТАБЛЕТКИ ПРОЛОНГИРОВАННОГО ДЕЙСТВИЯ
Консультация по дозировке, побочным эффектам, взаимодействиям, противопоказаниям и продлению рецепта на ТАПЕНТАДОЛ ТЕВА 100 мг ТАБЛЕТКИ ПРОЛОНГИРОВАННОГО ДЕЙСТВИЯ – по решению врача и с учетом местных правил.














