
TAPENTADOL RETARD STADA 100 MG COMPRIMIDOS DE LIBERACION PROLONGADA EFG

Cómo usar TAPENTADOL RETARD STADA 100 MG COMPRIMIDOS DE LIBERACION PROLONGADA EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Tapentadol retardStada25 mg comprimidos de liberación prolongada EFG
Tapentadol retard Stada 50 mg comprimidos de liberación prolongada EFG
Tapentadol retard Stada 100 mg comprimidos de liberación prolongada EFG
Tapentadol retard Stada 150 mg comprimidos de liberación prolongada EFG
Tapentadol retard Stada 200 mg comprimidos de liberación prolongada EFG
Tapentadol retard Stada 250 mg comprimidos de liberación prolongada EFG
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Tapentadol retard Stada y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Tapentadol retard Stada
- Cómo tomar Tapentadol retard Stada
- Posibles efectos adversos
- Conservación de Tapentadol retard Stada
- Contenido del envase e información adicional
1. Qué es Tapentadol retard Stada y para qué se utiliza
Tapentadol - el principio activo de Tapentadol retard Stada - es un analgésico potente que pertenece a la clase de los opioides. Tapentadol se utiliza para el tratamiento del dolor crónico intenso en adultos, que solo se puede tratar adecuadamente con un analgésico opioide.
2. Qué necesita saber antes de empezar a tomar Tapentadol retard Stada
No tome Tapentadol retard Stada
- si es alérgico a tapentadol o a alguno de los demás componentes de este medicamento (incluidos en la sección 6)
- si tiene asma o si su respiración es lenta o poco profunda hasta niveles peligrosos (depresión respiratoria, hipercapnia)
- si padece parálisis del intestino
- si sufre intoxicación aguda con alcohol, pastillas para dormir, analgésicos u otros medicamentos psicotrópicos (medicamentos que afectan el estado de ánimo y las emociones) (ver “Otros medicamentos y Tapentadol retard Stada”)
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a tomar Tapentadol retard Stada:
- si su respiración es lenta o poco profunda,
- si tiene un aumento de la presión cerebral o alteración de la consciencia hasta incluso el coma,
- si ha tenido un traumatismo craneal o tumores cerebrales,
- si tiene una enfermedad hepática o renal (ver “Cómo tomar Tapentadol retard Stada”),
- si tiene una enfermedad del páncreas o de las vías biliares, incluyendo pancreatitis,
- si está tomando medicamentos denominados agonistas/antagonistas opioides mixtos (por ejemplo: pentazocina, nalbufina) o agonistas parciales de los receptores opioides mu (por ejemplo: buprenorfina),
- si es propenso a padecer epilepsia o ataques o si está tomando otros medicamentos con riesgo conocido de aumentar las convulsiones, ya que el riesgo de estas crisis puede aumentar,
- si usted o algún miembro de su familia tiene antecedentes de abuso o dependencia del alcohol, de medicamentos de venta con receta o de sustancias ilícitas ("adicción"),
- si fuma,
- si alguna vez ha tenido problemas con su estado de ánimo (depresión, ansiedad o un trastorno de la personalidad) o ha recibido tratamiento de un psiquiatra para otras enfermedades mentales.
Este medicamento contiene tapentadol, que es un medicamento opioide. El uso repetido de analgésicos opioides puede restarle eficacia al medicamento (puede acostumbrarse a él). También puede conllevar dependencia y abuso, lo que puede dar lugar a una sobredosis potencialmente mortal. Es importante que informe a su médico si piensa que puede haber desarrollado dependencia a tapentadol. Su uso (incluso a dosis terapéuticas) puede provocar una dependencia física, lo que quizá provoque que sufra efectos de abstinencia y una reaparición de sus problemas si deja de tomar repentinamente este tratamiento farmacológico.
Tapentadol puede producir adicción a nivel físico y psicológico. Si tiene tendencia al abuso de medicamentos o si tiene dependencia a los medicamentos, debe tomar estos comprimidos unicamente durante periodos cortos de tiempo y bajo una supervisión médica estricta.
Trastornos respiratorios relacionados con el sueño
Tapentadol puede causar trastornos respiratorios relacionados con el sueño como apnea del sueño (pausa de la respiración durante el sueño) e hipoxemia relacionada con el sueño (niveles bajos de oxígeno en la sangre). Los síntomas pueden incluir pausa en la respiración durante el sueño, despertares nocturnos debido a la dificultad para respirar, dificultad para mantener el sueño o somnolencia excesiva durante el día. Si usted u otra persona observa estos síntomas, consulte a su médico. Su médico puede considerar disminuir la dosis.
Otros medicamentos y Tapentadol retard Stada
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
- El riesgo de efectos secundarios aumenta si está tomando medicamentos que puedan provocar convulsiones (ataques), como es el caso de ciertos antidepresivos o antipsicóticos. El riesgo de crisis convulsivas aumenta si toma tapentadol de forma simultánea a estos medicamentos. Su médico le dirá si tapentadol retard es adecuado para usted.
- El uso concomitante de tapentadol y medicamentos sedantes como las benzodiacepinas o medicamentos relacionados [ciertos comprimidos para dormir o tranquilizantes (p. ej., barbitúricos) o analgésicos como los opioides, la morfina y la codeína (también como medicamento para la tos), antipsicóticos, antihistamínicos H1, alcohol] aumenta el riesgo de somnolencia, dificultades respiratorias (depresión respiratoria), coma y puede ser potencialmente mortal. Debido a esto, solo se debe considerar el uso concomitante cuando no son posibles otras opciones de tratamiento. Sin embargo, si su médico le receta tapentadol retard junto con medicamentos sedantes, deberá limitarle la dosis y la duración del tratamiento concomitante. El uso concomitante de opioides y fármacos utilizados para el tratamiento de la epilepsia, el dolor nervioso o la ansiedad (gabapentina y pregabalina) aumenta el riesgo de sobredosis por opioides, de depresión respiratoria y puede ser potencialmente mortal. Informe a su médico si está tomando gabapentina o pregabalina o cualquier otro medicamento sedante y siga de forma rigurosa la recomendación de dosis de su médico. Puede ser útil informar a sus amigos y familiares sobre los signos y síntomas indicados anteriormente. Contacte con su médico si experimenta alguno de estos síntomas.
- Si está tomando un tipo de medicamento que afecta los niveles de serotonina (p. ej. ciertos medicamentos para tratar la depresión), hable con su médico antes de tomar tapentadol retard ya que ha habido casos de "síndrome serotoninérgico". El síndrome serotoninérgico es un trastorno raro, pero potencialmente mortal. Los signos pueden incluir contracciones involuntarias, rítmicas de los músculos, incluyendo los músculos que controlan el movimiento del ojo, agitación, sudoración excesiva, temblores, reflejos exagerados, aumento de la tensión muscular y temperatura corporal por encima de 38 ºC. Su médico le puede aconsejar.
- No se ha estudiado la administración conjunta de tapentadol junto con otros tipos de medicamentos denominados agonistas/antagonistas mixtos de los receptores de los opioides mu (p. ej., pentazocina, nalbufina) ni con agonistas parciales de los opioides mu (p. ej., buprenorfina). Es posible que tapentadol no funcione tan bien si se administra junto con alguno de estos medicamentos. Informe a su médico si está siendo tratado actualmente con uno de estos medicamentos.
- La administración de tapentadol junto con inhibidores o inductores potentes (p. ej., rifampicina, fenobarbital o hierba de San Juan) de determinadas enzimas necesarias para eliminar el tapentadol de su cuerpo, puede influir en la eficacia de tapentadol o puede causar efectos adversos, especialmente cuando se inicia o se suspende este otro tipo de medicación. Por favor, mantenga informado a su médico sobre todos los medicamentos que está tomando.
- Tapentadol no debe tomarse junto con inhibidores de la MAO (ciertos medicamentos para el tratamiento de la depresión). Informe a su médico si está tomando inhibidores de la MAO o si los ha tomado en los últimos 14 días.
Toma de Tapentadol retard Stada con alimentos, bebidas y alcohol
No consuma alcohol mientras esté tomando tapentadol, porque algunos efectos secundarios como la somnolencia pueden aumentar. La toma de alimentos no influye en el efecto de este medicamento.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No tome este medicamento:
- si está embarazada, a menos que su médico se lo haya indicado, ya que si se usa durante periodos prolongados durante el embarazo, tapentadol puede provocar síntomas de abstinencia en el recién nacido, que puede poner en peligro la vida del recién nacido si no es detectado y tratado por un médico.
- durante la lactancia, porque puede excretarse por la leche materna.
No se recomienda la toma de tapentadol:
- durante el parto, porque puede producir respiración lenta o poco profunda hasta niveles peligrosos (depresión respiratoria) en el recién nacido.
Conducción y uso de máquinas
Pregunte a su médico si puede conducir o utilizar máquinas durante el tratamiento con Tapentadol retard. Es importante que antes de conducir o utilizar máquinas, observe como le afecta este medicamento. No conduzca ni utilice máquinas si siente sueño, mareo, tiene visión borrosa o ve doble, o tiene dificultad para concentrarse. Tenga especial cuidado al inicio del tratamiento, tras un cambio de la dosis y al administrarlo conjuntamente con alcohol o tranquilizantes.
3. Cómo tomar Tapentadol retard Stada
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Su médico ajustará la dosis en función de la intensidad de su dolor y de su sensibilidad personal al dolor. Generalmente, deberá tomarse la dosis más baja para aliviar el dolor.
Adultos
La dosis recomendada inicial es de 50 mg dos veces al día, aproximadamente cada 12 horas.
No se recomiendan dosis diarias totales superiores a 500 mg de tapentadol.
Puede que su médico le prescriba una dosis o una pauta posológica diferente y más adecuada si es necesario. Si cree que el efecto de estos comprimidos es demasiado fuerte o débil, hable con su médico o farmacéutico.
Pacientes de edad avanzada
En los pacientes de edad avanzada (de más de 65 años de edad) habitualmente no es necesario ajustar la dosis. No obstante, la eliminación de tapentadol puede retrasarse en algunos pacientes de este grupo de edad. Si esto le sucede a usted, puede que su médico le prescriba una pauta posológica diferente.
Enfermedades hepáticas y renales (insuficiencia)
Los pacientes con problemas hepáticos graves no deben tomar estos comprimidos. Si tiene problemas moderados, su médico le prescribirá una pauta posológica diferente. En caso de problemas hepáticos leves, no es necesario ajustar la dosis.
Los pacientes con problemas renales graves no deben tomar estos comprimidos. En caso de problemas renales leves o moderados, no es necesario ajustar la dosis.
Uso en niños y adolescentes
Tapentadol retard no está indicado en niños y adolescentes de menos de 18 años.
Cómo y cuándo tomar Tapentadol retard Stada
Tapentadol retard debe tomarse por vía oral.
Trague siempre el comprimido con una cantidad de líquido suficiente. No lo mastique ni lo triture, esto podría conducir a una sobredosis porque el principio activo se liberará en su cuerpo demasiado deprisa. Puede tomar los comprimidos con el estómago vacío o con las comidas.
El comprimido se puede dividir en dosis iguales.
Puede que el recubrimiento del comprimido no se digiera completamente y, por lo tanto, se vea en las heces. Esto no debe preocuparle ya que el medicamento (principio activo) del comprimido ya habrá sido absorbido por el cuerpo y lo que usted ve es únicamente el recubrimiento vacío.
Instrucciones de apertura del blíster
Este medicamento está envasado en blísters precortados unidosis de seguridad a prueba de niños. No puede presionar hacia fuera el comprimido a través del blíster. Por favor, observe las siguientes instrucciones de apertura del blíster:
- Corte una dosis a lo largo de las líneas precortadas del blíster.
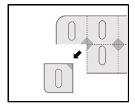
- Localice la zona no sellada que se encuentra donde las líneas precortadas se cruzan.

- Tire de la zona no sellada para despegar la lámina

Durante cuánto tiempo debe tomar Tapentadol retard Stada
No tome los comprimidos durante más tiempo del que le ha indicado su médico.
Si toma más Tapentadol retard Stada del que debe
Tras tomar dosis muy altas, puede presentar alguno de los siguientes efectos:
- pupilas muy reducidas
- vómitos
- disminución de la presión arterial
- latidos cardíacos acelerados
- desmayo, alteración de la consciencia o coma (pérdida profunda de la consciencia)
- crisis epilépticas
- respiración lenta o poco profunda hasta niveles peligrosos o parada respiratoria.
Si le sucede alguno de estos efectos, debe llamar a un médico inmediatamente.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20 indicando el medicamento y la cantidad utilizada. Se recomienda llevar el envase y el prospecto del medicamento al profesional sanitario.
Si olvidó tomarTapentadol retard Stada
Si olvida tomar un comprimido, es probable que vuelva a sentir dolor. No tome una dosis doble para compensar las dosis olvidadas, simplemente continúe tomando los comprimidos como antes.
Si interrumpe el tratamiento con Tapentadol retard Stada
Si interrumpe o deja de tomar el tratamiento demasiado pronto, es probable que vuelva a sentir dolor. Si desea interrumpir el tratamiento, consulte a su médico antes de hacerlo.
Generalmente, no se presentan síntomas de abstinencia tras interrumpir el tratamiento, sin embargo, en raras ocasiones, personas que han tomado los comprimidos durante bastante tiempo se sienten mal si dejan de tomarlos de repente.
Los síntomas pueden ser:
- inquietud, ojos llorosos, moqueo nasal, bostezos, sudoración, escalofríos, dolor muscular y pupilas dilatadas,
- irritabilidad, ansiedad, dolor de espalda, dolor articular, debilidad, calambres abdominales,
dificultades para dormir, náuseas, pérdida del apetito, vómitos, diarrea y aumentos de la presión arterial, la frecuencia respiratoria o la frecuencia cardíaca.
Si experimenta alguno de estos síntomas tras interrumpir el tratamiento, por favor, consulte a su médico.
No debe interrumpir bruscamente este medicamento, salvo que su médico así se lo indique. Si su médico quiere que deje de tomar estos comprimidos, le indicará cómo debe hacerlo, lo cual podría implicar una reducción gradual de la dosis.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Efectos adversos o síntomas importantes a los que estar atento y qué hacer si usted está afectado por los mismos:
- Este medicamento puede causar reacciones alérgicas. Los síntomas pueden consistir en sibilancias, dificultad para respirar, inflamación de los párpados, cara o labios, erupción cutánea o picor, especialmente si afectan a todo el cuerpo.
- Otro efecto adverso grave consiste en respirar más despacio o más débilmente de lo normal. Ocurre mayoritariamente en pacientes de edad avanzada o en pacientes debilitados.
Si presenta alguno de estos importantes síntomas consulte a su médico inmediatamente.
Otros efectos adversos que se pueden presentar:
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas)
- náuseas, estreñimiento
- mareos, somnolencia, dolor de cabeza
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- disminución del apetito, ansiedad, depresión del estado de ánimo, dificultades para dormir, nerviosismo, inquietud, alteraciones en la atención,
- temblores, tics musculares,
- sofocos,
- falta de aliento,
- vómitos, diarrea, indigestión,
- picores, aumento de la sudoración, erupciones cutáneas,
- sensación de debilidad, cansancio, sensación de cambio en la temperatura corporal, sequedad de las mucosas, acumulación de agua en los tejidos (edema).
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas)
- reacción alérgica a medicamentos (incluyendo hinchazón bajo la piel, habón urticarial y en casos graves dificultad para respirar, disminución de la presión arterial, colapso o shock),
- pérdida de peso,
- desorientación, confusión, excitabilidad (agitación), alteraciones de la percepción, alteraciones del sueño, estado de ánimo eufórico, depresión del nivel de consciencia, deterioro de la memoria, deterioro mental,
- desvanecimiento, sedación, trastornos del equilibrio, dificultades para hablar, entumecimiento, sensaciones anormales en la piel (por ejemplo, hormigueo, picor),
- alteración de la visión,
- latidos cardíacos acelerados, latidos cardíacos lentos, palpitaciones, disminución de la presión arterial,
- malestar abdominal,
- sarpullido,
- retraso en el paso de la orina, orinar frecuentemente,
- disfunción sexual,
- síndrome de abstinencia a fármacos (ver “Si interrumpe el tratamiento con Tapentadol retard Stada”), sensación de malestar, irritabilidad.
Raros (pueden afectar hasta 1 de cada 1.000 personas)
- dependencia al fármaco, alteración de pensamiento, crisis epilépticas, sensación de estar a punto de desmayarse, coordinación alterada,
- respiración lenta o poco profunda hasta niveles peligrosos (depresión respiratoria),
- alteración del vaciado gástrico,
- sensación de embriaguez, sensación de relajación.
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles)
- delirio
En general, la posibilidad de tener pensamientos y comportamientos suicidas aumenta en pacientes con
dolor crónico. Además, algunos medicamentos para tratar la depresión (con impacto en el sistema neurotransmisor del cerebro) pueden aumentar ese riesgo, especialmente al inicio del tratamiento. Aunque tapentadol también afecte a los neurotransmisores, a través de la experiencia en pacientes no se ha probado que aumente este riesgo.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Tapentadol retard Stada
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y el blíster después de CAD. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere condiciones especiales de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Tapentadol retard Stada
El principio activo es tapentadol.
Tapentadol retard Stada 25 mg comprimidos de liberación prolongada EFG
Cada comprimido de liberación prolongada contiene tapentadol fosfato equivalente a 25 mg de tapentadol.
Tapentadol retard Stada 50 mg comprimidos de liberación prolongada EFG
Cada comprimido de liberación prolongada contiene tapentadol fosfato equivalente a 50 mg de tapentadol.
Tapentadol retard Stada 100 mg comprimidos de liberación prolongada EFG
Cada comprimido de liberación prolongada contiene tapentadol fosfato equivalente a 100 mg de tapentadol.
Tapentadol retard Stada 150 mg comprimidos de liberación prolongada EFG
Cada comprimido de liberación prolongada contiene tapentadol fosfato equivalente a 150 mg de tapentadol.
Tapentadol retard Stada 200 mg comprimidos de liberación prolongada EFG
Cada comprimido de liberación prolongada contiene tapentadol fosfato equivalente a 200 mg de tapentadol.
Tapentadol retard Stada 250 mg comprimidos de liberación prolongada EFG
Cada comprimido de liberación prolongada contiene tapentadol fosfato equivalente a 250 mg de tapentadol.
Los demás componentes (excipientes) son:
Núcleo del comprimido: celulosa microcristalina (E460), hipromelosa (E464), sílice coloidal anhidra (E551), estearato de magnesio.
Recubrimiento del comprimido: hipromelosa (E464), glicerol (E422), talco (E553b), celulosa microcristalina (E460), dióxido de titanio (E171), óxido de hierro rojo (solo en las dosis de 25, 100, 150, 200 y 250 mg) (E172), óxido de hierro amarillo (solo en las dosis de 25, 100 y 200 mg) (E172), óxido de hierro negro (solo en las dosis de 25, 100, 150, 200 y 250 mg) (E172).
Aspecto del producto y contenido del envase
Tapentadol retard Stada 25 mg comprimidos de liberación prolongada EFG son comprimidos de color marronoso, oblongos, biconvexos (6 mm x 12 mm) y ranurados en ambas caras.
El comprimido se puede dividir en dosis iguales.
Tapentadol retard Stada 50 mg comprimidos de liberación prolongada EFG son comprimidos de color blanco, oblongos, biconvexos (6 mm x 13 mm) y ranurados en ambas caras.
El comprimido se puede dividir en dosis iguales.
Tapentadol retard Stada 100 mg comprimidos de liberación prolongada EFG son comprimidos de color amarillento, oblongos, biconvexos (7 mm x 14 mm) y ranurados en ambas caras.
El comprimido se puede dividir en dosis iguales.
Tapentadol retard Stada 150 mg comprimidos de liberación prolongada EFG son comprimidos de color rojizo brillante, oblongos, biconvexos (7 mm x 15 mm) y ranurados en ambas caras.
El comprimido se puede dividir en dosis iguales.
Tapentadol retard Stada 200 mg comprimidos de liberación prolongada EFG son comprimidos de color amarillo, oblongos, biconvexos (8 mm x 16 mm) y ranurados en ambas caras.
El comprimido se puede dividir en dosis iguales.
Tapentadol retard Stada 250 mg comprimidos de liberación prolongada EFG son comprimidos de color marrón rojizo, oblongos, biconvexos (9 mm x 18 mm) y ranurados en ambas caras.
El comprimido se puede dividir en dosis iguales.
Tapentadol retard Stada está disponible en los siguientes formatos:
Tapentadol retard Stada 25 mg comprimidos de liberación prolongada EFG
20x1, 30x1, 40x1, 50x1, 54x1, 60x1 o 100x1 comprimidos en blísteres precortados unidosis de seguridad a prueba de niños.
Tapentadol retard Stada 50, 100, 150, 200, 250 mg comprimidos de liberación prolongada EFG
20x1, 24x1, 30x1, 50x1, 54x1, 60x1 o 100x1 comprimidos en blísteres precortados unidosis de seguridad a prueba de niños.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Laboratorio STADA, S.L.
Frederic Mompou, 5
08960 Sant Just Desvern (Barcelona)
España
Responsable de la fabricación
Develco Pharma GmbH
Grienmatt 27
79650 Schopfheim
Alemania
o
STADA Arzneimittel AG
Stadastrasse 2 – 18
61118 Bad Vilbel
Alemania
o
Centrafarm Services B.V.,
Van de Reijtstraat 31-E,
4814 NE Breda,
Países Bajos
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Alemania | Tapentadol AL 25 mg Retardtabletten Tapentadol AL 50 mg Retardtabletten Tapentadol AL 100 mg Retardtabletten Tapentadol AL 150 mg Retardtabletten Tapentadol AL 200 mg Retardtabletten Tapentadol AL 250 mg Retardtabletten |
Croacia | TAPISTA |
Dinamarca | Tapentadol STADA |
Eslovaquia | Tapestad retard 50 mg, 100 mg, 150 mg, 200 mg, 250 mg |
España | Tapentadol retard STADA 25 mg comprimidos de liberación prolongada EFG Tapentadol retard STADA 50 mg comprimidos de liberación prolongada EFG Tapentadol retard STADA 100 mg comprimidos de liberación prolongada EFG Tapentadol retard STADA 150 mg comprimidos de liberación prolongada EFG Tapentadol retard STADA 200 mg comprimidos de liberación prolongada EFG Tapentadol retard STADA 250 mg comprimidos de liberación prolongada EFG |
Islandia | Tapentadol STADA 25 mg, 50 mg, 100 mg, 150 mg, 200 mg, 250 mg; forðahylki |
Italia | Mudol |
Noruega | Tapentadol STADA |
Países Bajos | Tapentadol retard CF 25 mg, tabletten met verlengde afgifte Tapentadol retard CF 50 mg, tabletten met verlengde afgifte Tapentadol retard CF 100 mg, tabletten met verlengde afgifte Tapentadol retard CF 150 mg, tabletten met verlengde afgifte Tapentadol retard CF 200 mg, tabletten met verlengde afgifte Tapentadol retard CF 250 mg, tabletten met verlengde afgifte |
Polonia | BINATTA |
República Checa | Taxemba |
Suecia | Tapentadol Depot STADA 25 mg, 50 mg, 100 mg, 150 mg, 200 mg, 250 mg; depottabletter |
Fecha de la última revisión de este prospecto:septiembre 2022.
La información detallada y actualizada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es/).
- País de registro
- Precio medio en farmacia44.02 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TAPENTADOL RETARD STADA 100 MG COMPRIMIDOS DE LIBERACION PROLONGADA EFGForma farmacéutica: COMPRIMIDO LIBERACION MODIFICADA, 100 mgPrincipio activo: tapentadolFabricante: Sandoz Farmaceutica S.A.Requiere recetaForma farmacéutica: COMPRIMIDO LIBERACION MODIFICADA, 150 mgPrincipio activo: tapentadolFabricante: Sandoz Farmaceutica S.A.Requiere recetaForma farmacéutica: COMPRIMIDO LIBERACION MODIFICADA, 200 mgPrincipio activo: tapentadolFabricante: Sandoz Farmaceutica S.A.Requiere receta
Médicos online para TAPENTADOL RETARD STADA 100 MG COMPRIMIDOS DE LIBERACION PROLONGADA EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TAPENTADOL RETARD STADA 100 MG COMPRIMIDOS DE LIBERACION PROLONGADA EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















