TAFLOTAN 15 MICROGRAMOS/ML COLIRIO EN SOLUCION


Cómo usar TAFLOTAN 15 MICROGRAMOS/ML COLIRIO EN SOLUCION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Taflotan 15 microgramos/ml colirio en solución
Tafluprost
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Taflotan y para qué se utiliza
- Qué necesita saber antes de empezar a usar Taflotan
- Cómo usar Taflotan
- Posibles efectos adversos
- Conservación de Taflotan
- Contenido del envase e información adicional
1. Qué es Taflotan y para qué se utiliza
¿Qué tipo de medicamento es y cómo funciona?
Taflotan colirio contiene tafluprost, que pertenece a un grupo de medicamentos llamados análogos de la prostaglandina. Taflotan reduce la presión ocular. Se utiliza cuando la presión en el interior del ojo es demasiado alta.
¿Para qué es este medicamento?
Taflotan se emplea para tratar un tipo de glaucoma llamado de ángulo abierto, y también una enfermedad conocida como hipertensión ocular en adultos. Ambos trastornos están ligados a un aumento de la presión del interior del ojo y, a la larga, pueden afectar a la visión.
2. Qué necesita saber antes de empezar a usar Taflotan
No use Taflotan
- Si es alérgico al tafluprost o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar Taflotan
Por favor tenga en cuenta queTaflotan puede tener los siguientes efectos y algunos de ellos pueden ser permanentes:
- Taflotan puede aumentar la longitud, el grosor, el color y/o la cantidad de pestañas, y puede causar un crecimiento anómalo de vello en los párpados.
- Taflotan puede causar un oscurecimiento del color de la piel alrededor de los ojos. Seque el exceso de solución de la piel. Esto reducirá el riesgo de oscurecimiento de la piel.
- Taflotan puede cambiar el color del iris (la parte coloreada del ojo). Si Taflotan se emplea sólo en un ojo, éste puede volverse de un color diferente al del ojo no tratado de manera permanente.
- Taflotan puede causar crecimiento de pelo en zonas dónde la solución entra en contacto con la superficie de la piel repetidamente.
Informe a su médico:
- si tiene algún problema en el riñón.
- si tiene algún problema en el hígado.
- si tiene asma.
- si tiene otras enfermedades oculares.
Niños y adolescentes
Taflotan no está recomendado para uso en niños y adolescentes menores de 18 años de edad, debido a una falta de datos sobre la seguridad y la eficacia.
Otros medicamentos y Taflotan
Comunique a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Si usa otros medicamentos en el ojo, espere por lo menos 5 minutos después de aplicarse Taflotan y antes de usar el otro medicamento.
Embarazo, lactancia y fertilidad
Si puede quedarse embarazada, debe emplear un método anticonceptivo eficaz durante el tratamiento con Taflotan. No use Taflotan si está embarazada. No debería usar Taflotan si está en periodo de lactancia. Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
Taflotan tiene poca influencia sobre la capacidad para conducir y utilizar máquinas. Tras la aplicación de Taflotan puede notar la visión borrosa durante un tiempo. No conduzca ni utilice ninguna herramienta o máquina hasta que la visión sea clara.
Taflotan contiene fosfatos
Este medicamento contiene aproximadamente 0,04 mg de fosfatos en cada gota, equivalente a 1,2 mg/ml. Si sufre de daño grave en la córnea (la capa trasparente de la parte frontal del ojo) el tratamiento con fosfatos, en casos muy raros, puede provocar visión borrosa por acumulación de calcio.
3. Cómo usar Taflotan
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada es de1 gota de Taflotan en un ojo o en ambos, una vez al día, por la noche. No instile más gotas ni lo utilice con mayor frecuencia que la indicada por su médico.
Esto puede hacer que Taflotan sea menos eficaz.
Utilice sólo Taflotan en ambos ojos si su médico se lo indica.
Para uso sólo como colirio. No ingerir.
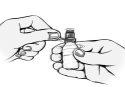
Instrucciones de uso:
Cuando lo utilice por primera vez, antes de aplicar una gota en el ojo, primero debe practicar el uso del frasco, oprimiéndolo lentamente para dejar caer una gota fuera del ojo.
Cuando esté seguro de poder aplicar una única gota cada vez, elija la posición más cómoda para la instilación de las gotas (puede sentarse, tumbarse sobre su espalda, o quedarse levantado delante de un espejo).
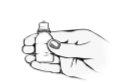
Al comenzar un frasco nuevo:
No utilice el frasco o si el anillo de plástico alrededor del cuello del frasco no está o está roto. Escriba la fecha en que abrió el frasco en el espacio reservado para la fecha, en la caja exterior.
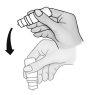
Cada vez que utilice Taflotan:
| |
|
|
| |
| |
|
|
Tenga especial cuidado en evitar que la punta del frasco cuentagotas toque su ojo, la piel alrededor del ojo o sus dedos para prevenir la potencial contaminación de la solución. | |
|
|
|
|
| |
|
|
Quedará un volumen residual de aproximadamente 1 ml, que no se debe administrar. No intente vaciar el frasco.
Si una gota no cae en el ojo, inténtelo de nuevo.
Si su médico le ha dicho que use colirio en ambos ojos, repita los pasos 6 a 9 en el otro ojo.
Si usa otros medicamentos en el ojo,espere por lo menos cinco minutos después de aplicarse Taflotan y antes de usar el otro medicamento.
Si usa más Taflotan del que debe,es poco probable que le cause un daño grave. Aplíquese la siguiente dosis a la hora habitual.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20.
Si olvidó usar Taflotan,aplíquese una sola gota en cuanto se acuerde, y vuelva a su horario normal. No use una dosis doble para compensar las dosis olvidadas.
No deje de usar Taflotan sin consultar a su médico. Si interrumpe el tratamiento con Taflotan,la presión dentro del ojo aumentará de nuevo. Esto puede causar una lesión permanente en el ojo.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran. La mayoría de los efectos adversos no son graves.
Efectos adversos frecuentes
Los siguientes efectos pueden afectar hasta 1 de cada 10 personas:
Efectos en el sistema nervioso:
- cefalea
Efectos en el ojo:
- picor en los ojos
- irritación de los ojos
- dolor de ojo
- enrojecimiento del ojo
- cambios en la longitud, el grosor y la cantidad de pestañas
- sequedad en el ojo
- sensación de tener un cuerpo extraño en el ojo
- cambio de color de las pestañas
- enrojecimiento de los párpados
- pequeñas zonas de inflamación en puntos en la superficie del ojo
- sensibilidad a la luz
- ojos llorosos
- visión borrosa
- disminución de la capacidad visual para ver detalles
- cambio de color del iris (puede ser permanente)
Efectos adversos poco frecuentes
Los siguientes efectos pueden afectar hasta 1 de cada 100 personas:
Efectos en el ojo:
- cambio de color de la piel alrededor de los ojos
- hinchazón de los párpados
- ojos cansados
- hinchazón de las membranas superficiales del ojo
- ojos llorosos
- inflamación de los párpados
- signos de inflamación en el interior del ojo
- molestias en el ojo
- pigmentación de las membranas superficiales del ojo
- folículos en las membranas superficiales del ojo
- inflamación alérgica
- sensación anormal en el ojo
Efectos en la piel y en los tejidos debajo de la piel:
- crecimiento no habitual de vello en los párpados
Frecuencia no conocida: no puede estimarse a partir de los datos disponibles
Efectos en el ojo:
- inflamación del iris/úvea (capa central del ojo)
- ojos hundidos
- edema macular/edema macular cistoide (inflamación de la retina dentro del ojo llevando al empeoramiento de la visión).
Efectos en el sistema respiratorio:
- empeoramiento del asma, respiración difícil
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Taflotan
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta del frasco y en la caja, después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2ºC y 8 ºC). No congelar.
Tras la apertura del frasco, conservar por debajo de 25ºC.
Conservar en el embalaje original para protegerlo de la luz.
Debe desechar el frasco 3 meses después de abrirlo por primera vez, para evitar infecciones, y utilizar un frasco nuevo.El frasco con un volumen de llenado de 3 ml está destinado para un período de uso de 1 mes, el frasco con un volumen de llenado de 5 ml para 2 meses y el frasco con un volumen de llenado de 7 ml para 3 meses.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Taflotan
- El principio activoes tafluprost. 1 ml de solución contiene 15 microgramos de tafluprost. 1 gota contiene alrededor de 0,45 microgramos de tafluprost.
- Los demás componentesson glicerol, dihidrógenofosfato de sodio dihidrato, edetato de disodio, polisorbato 80 y agua para preparaciones inyectables. Se añade ácido clorhídrico y/o hidróxido de sodio para ajustar el pH.
Aspecto del producto y contenido del envase
Taflotan es un líquido (solución) transparente e incoloro, prácticamente libre de partículas visibles. Se presenta en una caja que contiene 1 frasco transparente, de plástico, con 3 ml, 5 ml o 7 ml, o 3 frascos transparentes con 3 ml de solución en cada frasco. Los frascos de plástico están cerrados con tapones.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
Santen Oy
Niittyhaankatu 20
33720 Tampere
Finlandia
Responsable de la fabricación:
Santen Oy
Kelloportinkatu 1
33100 Tampere
Finlandia
O
Tubilux Pharma, S.p.A.
Via Costarica 20/22
00071 Pomezi, Roma
Italia
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Santen Pharmaceutical Spain, S.L.
Acanto, 22, 7º planta
28045 Madrid
Tel.: 91 414 24 85
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo y en el Reino Unido (Irlande del Norte) con los siguientes nombres:
Alemania | TAFLOTAN sine |
Dinamarca, Finlandia, Islandia, Noruega, Suecia | Taflotan sine |
Bulgaria, Chipre, República Checa, Estonia, Grecia, Hungría, Letonia, Lituania, Portugal, Eslovaquia, España | Taflotan |
Polonia | Taflotan Multi |
Austria, Bélgica, Croacia, Irlanda, Luxemburgo, Países Bajos, Rumania, Eslovenia, Reino Unido (Irlanda del Norte) | Saflutan |
Italia | Safluround |
Fecha de la última revisión de este prospecto:Junio 2023
La información detallada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (http://www.aemps.gob.es/)
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a TAFLOTAN 15 MICROGRAMOS/ML COLIRIO EN SOLUCIONForma farmacéutica: COLIRIO, 15 µg/mlPrincipio activo: TafluprostFabricante: Santen OyRequiere recetaForma farmacéutica: COLIRIO, 0.3 mg/mlPrincipio activo: BimatoprostFabricante: Tiedra Farmaceutica S.L.Requiere recetaForma farmacéutica: COLIRIO, 0.3 mg/mlPrincipio activo: BimatoprostFabricante: Sifi S.P.A.Requiere receta
Médicos online para TAFLOTAN 15 MICROGRAMOS/ML COLIRIO EN SOLUCION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de TAFLOTAN 15 MICROGRAMOS/ML COLIRIO EN SOLUCION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes