
AMIRIOX 0.3 mg/ml EYE DROPS SOLUTION

How to use AMIRIOX 0.3 mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET
Package Leaflet: Information for the User
Amiriox 0.3 mg/ml eye drops, solution
bimatoprost
Read the package leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is Amiriox and what is it used for
- What you need to know before you use Amiriox
- How to use Amiriox
- Possible side effects
- Storage of Amiriox
- Contents of the pack and other information
1. What is Amiriox and what is it used for
Amiriox contains the active substance bimatoprost, which is a medicine for glaucoma. It belongs to a group of medicines called prostamides.
Bimatoprost is used to reduce high pressure in the eye in adults. This medicine can be used alone or with other eye drops called beta-blockers that also reduce pressure.
This medicine works by increasing the amount of fluid that drains from the eye to prevent accumulation. This reduces the pressure inside the eye. If this pressure is not reduced, it could lead to a disease called glaucoma and damage your vision.
This medicine does not contain preservatives.
2. What you need to know before you use Amiriox
Do not useAmiriox
- if you are allergic to bimatoprost or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use bimatoprost.
Consult your doctor or pharmacist if:
- you have any respiratory problems
- you have liver or kidney problems
- you have had cataract surgery in the past
- you have low blood pressure or a slow heart rate
- you have had a viral infection or eye inflammation
During treatment, Amiriox may cause fat loss around the eye that can cause deepening of the eyelid sulcus, sinking of the eyes (enophthalmos), drooping of the eyelids (ptosis), stretching of the skin around the eye (involution of dermatocalasis), and the white part of the eye becomes more visible (inferior scleral exposure). The changes are usually mild, but if they worsen, they can affect your field of vision. The changes may disappear if you stop using Amiriox. Amiriox may also cause darkening and growth of eyelashes, as well as darkening of the skin around the eyelid. The color of the iris may darken. These changes may be permanent and more visible if only one eye is being treated.
If you have a history of contact hypersensitivity to silver, you should not use this medicine.
Children and adolescents
Bimatoprost has not been studied in children under 18 years of age and should not be used in patients under 18 years of age.
Other medicines and Amiriox
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
If you are using other eye drops, wait at least 5 minutes between administering bimatoprost and the other eye drops. Ophthalmic ointments should be administered last.
Pregnancy, breastfeeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Bimatoprost may pass into breast milk, so you should not breastfeed while using this medicine.
Driving and using machines
Blurred vision may occur for a short time after using this medicine. Do not drive or use machines until your vision is clear.
Amiriox contains phosphates
This medicine contains 0.95 mg of phosphates per ml. If you have severe damage to the transparent layer in the front of the eye (cornea), treatment with phosphates, in very rare cases, may cause blurred vision due to calcium accumulation.
3. How to use Amiriox
Follow the instructions for administration of this medicine exactly as told by your doctor or pharmacist. If you are not sure, talk to your doctor or pharmacist.
Bimatoprost should only be used in the eye. The recommended dose is one drop of bimatoprost in each eye that needs treatment, once a day, in the evening.
Bimatoprost has not been studied in patients wearing contact lenses. Remove contact lenses before application and wait at least 15 minutes before putting them back in.
If you use bimatoprost with another eye medication, wait at least 5 minutes between using bimatoprost and the other eye medication.
Do not use the medicine more than once a day, as this may reduce the effectiveness of the treatment.
This medicine is a sterile solution that does not contain preservatives. See section 6, Appearance ofAmirioxand pack contents.
Before administering the eye drops:
- When you first use it, practice using the dropper bottle by slowly squeezing until you drop one drop in the air, away from the eye.
- When you are sure you can administer one drop at a time, choose the position you find most comfortable for administering the drops (sitting, lying on your back, or standing in front of a mirror).
Instructions for use:
- Wash your hands carefully before using this medicine.
- If the packaging or bottle is damaged, do not use the medicine.
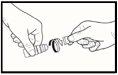
- When you use the medicine for the first time, unscrew the cap after making sure the ring seal has not been broken. You should feel a slight resistance until the security ring breaks (see drawing 1).
- If the security ring comes loose, discard it, as it may fall into the eye.
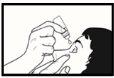
- Tilt your head back and gently pull the lower eyelid down to form a pocket between the eye and the eyelid (see drawing 2). Do not let the tip of the bottle touch the eye, eyelids, or fingers.
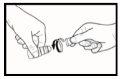
- Gently squeeze the bottle in the middle to release one drop into the eye (see drawing 3). There may be a delay of a few seconds between squeezing and the drop falling. Make sure not to squeeze the bottle too hard. If you are not sure how to use this medicine, talk to your doctor, pharmacist, or nurse.
- Keeping the eye closed, press with your finger against the corner of the closed eye (where the eye meets the nose) and hold for 2 minutes. This ensures that the drop is absorbed by the eye and helps prevent the medicine from reaching the rest of the body.
- Repeat steps 5, 6, and 7 in the other eye if your doctor has told you to do so.
- After use, shake the bottle once downwards, without touching the tip of the dropper, to remove any residual liquid from the tip. This will ensure the correct application of subsequent drops. Replace the cap on the bottle (see drawing 4).
If a drop does not reach the eye, try again.
To help prevent infections and avoid eye injuries, prevent the tip of the container
from touching the eye or any other surface. Replace the cap and close the container immediately
after use.

Drawing 1.Drawing 2.Drawing 3.Drawing 4.
If you use moreAmirioxthan you should
If you use more Amiriox than you should, it is unlikely to cause you any serious harm. Apply the next dose at the usual time. If you are concerned, talk to your doctor or pharmacist.
If you forget to useAmiriox
If you forget to apply this medicine, apply one drop as soon as you remember and then return to your usual routine. Do not apply a double dose to make up for the forgotten dose.
If you stop using Amiriox
This medicine should be used every day for it to work well. If you stop using this medicine, the pressure in the eye may increase, so consult your doctor before stopping treatment.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet.
Very common: may affect more than 1 in 10 people
Affecting the eye
- Mild redness (up to 24% of people)
- Fat loss in the eye area that can cause deepening of the eyelid sulcus, sinking of the eyes (enophthalmos), drooping of the eyelids (ptosis), stretching of the skin around the eye (involution of dermatocalasis), and the white part of the eye becomes more visible (inferior scleral exposure).
Common: may affect up to 1 in 10 people
Affecting the eye
- Small erosions on the surface of the eye, with or without inflammation
- Irritation
- Itching in the eyes
- Pain
- Dryness
- Feeling of having something in the eye
- Longer eyelashes
- Darker skin around the eye
- Red eyelids
Uncommon: may affect up to 1 in 100 people
Affecting the eye
- Tired eyes
- Sensitivity to light
- Darker iris
- Inflamed and itchy eyelids
- Excessive tearing
- Inflammation of the transparent layer covering the surface of the eye
- Blurred vision
Affecting the body
- Headaches
- Hair growth around the eye
Frequency not known: cannot be estimated from the available data
Affecting the eye
- Sticky eyes
- Eye discomfort
Affecting the body
- Asthma
- Worsening of asthma
- Worsening of chronic obstructive pulmonary disease (COPD)
- Breathing difficulties
- Symptoms of allergic reaction (inflammation, redness of the eye, and skin rash)
- Dizziness
- High blood pressure
- Discoloration of the skin (periocular)
In addition to the side effects of bimatoprost 0.3 mg/ml in single-dose preservative-free formulations, the following side effects have been observed with the multidose preserved formulation of bimatoprost 0.3 mg/ml and may occur in patients using bimatoprost 0.3 mg/ml in multidose preservative-free formulations:
- Burning sensation in the eye
- Allergic reaction in the eye
- Inflammation of the eyelids
- Difficulty seeing clearly
- Worsening of vision
- Darkening of eyelashes
- Bleeding in the back of the eye (retinal hemorrhage)
- Inflammation inside the eye
- Cystoid macular edema (inflammation of the retina inside the eye leading to worsening of vision)
- Inflammation of the iris
- Lid twitching
- Lid separating from the eye surface
- Nausea
- Redness of the skin around the eye
- Weakness
- Increased liver function test values
Other side effects reported with phosphate-containing eye drops
In very rare cases, some patients with severe damage to the transparent layer in the front of the eye (cornea) have developed cloudy patches in the cornea due to calcium accumulation during treatment.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Amiriox
This medicine does not require any special storage conditions.
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after “EXP”. The expiry date is the last day of the month shown.
After opening the bottle for the first time - store for 90 days at a temperature below 25°C.
Discard the bottle 90 days after opening it for the first time.
Medicines should not be disposed of via wastewater or household waste. Return the containers and any unused medicines to a pharmacy for disposal. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
Composition of Amiriox:
- The active substance is bimatoprost. One ml of solution contains 0.3 mg of bimatoprost.
- The other ingredients are sodium hydrogen phosphate dodecahydrate, citric acid monohydrate, sodium chloride, hydrochloric acid (for pH adjustment), water for injections.
Appearance of Amiriox and pack contents
Amiriox is a clear and transparent solution.
This medicine is available in 5 ml white LDPE bottles, with 3 ml of solution each, with a multidose HDPE dropper and a screw cap with a security seal.
The dropper has a silicone valve system that prevents contaminated liquid from flowing back into the bottle and allows filtered air to enter.
Pack sizes:
Boxes containing 1 or 3 bottles of 3 ml of solution each.
Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer
Marketing authorisation holder
SIFI S.p.A.
Via Ercole Patti 36
95025 Aci Sant'Antonio (CT)
Italy
Manufacturer
RAFARM S.A.
Thesi Pousi Xatzi Agiou Louka
190 02 Paiania
Greece
or
SIFI S.p.A.
Via Ercole Patti 36
95025 Aci Sant'Antonio (CT)
Italy
Local representative
SIFI IBÉRICA S.L.
C/ Poeta Joan Maragall, 47
Planta 4, Puerta 402
28020 Madrid - Spain
Date of last revision of this leaflet:August 2024
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es.
- Country of registration
- Average pharmacy price13.24 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AMIRIOX 0.3 mg/ml EYE DROPS SOLUTIONDosage form: EYEDROP, 0.3 mg/mlActive substance: bimatoprostManufacturer: Tiedra Farmaceutica S.L.Prescription requiredDosage form: EYEDROP, 0.3 mg/mlActive substance: bimatoprostManufacturer: Laboratorio Stada S.L.Prescription requiredDosage form: EYE DROP, 0.3 mg/mlActive substance: bimatoprostManufacturer: Laboratorios Salvat S.A.Prescription required
Online doctors for AMIRIOX 0.3 mg/ml EYE DROPS SOLUTION
Discuss questions about AMIRIOX 0.3 mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















