
SOMAVERT 25 MG POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE

Cómo usar SOMAVERT 25 MG POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
SOMAVERT 10 mg polvo y disolvente para solución inyectable
SOMAVERT 15 mg polvo y disolvente para solución inyectable
SOMAVERT 20 mg polvo y disolvente para solución inyectable
SOMAVERT 25mg polvo y disolvente para solución inyectable
SOMAVERT 30mg polvo y disolvente para solución inyectable
pegvisomant
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es SOMAVERT y para qué se utiliza
- Qué necesita saber antes de empezar a usar SOMAVERT
- Cómo usar SOMAVERT
- Posibles efectos adversos
- Conservación de SOMAVERT
- Contenido del envase e información adicional
1. Qué es SOMAVERT y para qué se utiliza
SOMAVERT se usa para el tratamiento de la acromegalia, un trastorno hormonal resultante del aumento de la secreción de la hormona de crecimiento (HC) y del IGF‑I (factores de crecimiento tipo insulina), y se caracteriza por sobrecrecimiento de los huesos, engrosamiento de los tejidos blandos, enfermedad del corazón y trastornos relacionados.
El principio activo de SOMAVERT, pegvisomant, es conocido como un antagonista del receptor de la hormona de crecimiento. Estas sustancias reducen la acción de la HC y los niveles de IGF‑I que circulan en sangre.
2. Qué necesita saber antes de empezar a usar SOMAVERT
No use SOMAVERT
- Si es alérgico a pegvisomant o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar SOMAVERT.
- Si nota trastornos en la visión o dolores de cabeza debe decírselo a su médico inmediatamente.
- Su médico o enfermera controlará los niveles de IGF‑I (factores de crecimiento tipo insulina) que circulan en sangre y si es necesario ajustará la dosis de SOMAVERT.
- Su médico debe también controlar su adenoma (tumor benigno).
- Su médico le realizará pruebas de función hepática antes de comenzar y durante el tratamiento con SOMAVERT. Si los resultados de estas pruebas no son normales, su médico le comentará las opciones de tratamiento. Una vez que el tratamiento comience, su médico o enfermera controlará los niveles de enzimas hepáticas en sangre cada 4‑6 semanas durante los primeros 6 meses de tratamiento con SOMAVERT. La administración de SOMAVERT se debe suspender si persisten los síntomas de la enfermedad hepática.
- Si es diabético, su médico puede necesitar ajustar la cantidad de insulina o de otros medicamentos que esté utilizando.
- La fertilidad en las pacientes puede aumentar a medida que mejora la enfermedad. No se recomienda utilizar este medicamento en mujeres embarazadas y se debe aconsejar a las mujeres en edad fértil que utilicen un método anticonceptivo. Ver también el apartado sobre Embarazo que aparece más adelante.
Otros medicamentos y SOMAVERT
Debe decirle a su médico si ha usado anteriormente otro medicamento para el tratamiento de la acromegalia o algún medicamento para el tratamiento de la diabetes.
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente cualquier otro medicamento. Como parte de su tratamiento, puede ser tratado con otros medicamentos. Es importante que siga utilizando todos los medicamentos incluido SOMAVERT a menos que reciba otra indicación por parte de su médico, farmacéutico o enfermero.
Embarazo, lactancia y fertilidad
No se recomienda utilizar SOMAVERT en mujeres embarazadas. Si es una mujer en edad fértil, debe utilizar un método anticonceptivo durante el tratamiento.
No se sabe si pegvisomant pasa a la leche materna. No deberá dar el pecho mientras esté tomando SOMAVERT a menos que lo haya discutido con su médico.
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
No se han realizado estudios de los efectos sobre la capacidad para conducir y utilizar máquinas.
SOMAVERT contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
3. Cómo usar SOMAVERT
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Su médico le administrará por vía subcutánea (debajo de la piel) una dosis inicial de 80 mg de pegvisomant. Después, la dosis diaria habitual es de 10 mg de pegvisomant administrada por inyección subcutánea (debajo de la piel).
Cada 4-6 semanas, su médico realizará los ajustes de dosis necesarios aumentando la dosis en 5 mg de pegvisomant por día, de acuerdo con sus niveles en sangre del ya mencionado IGF‑I, con el fin de obtener una respuesta terapéutica óptima.
Forma y vía de administración
SOMAVERT se inyecta bajo la piel. La inyección puede ponérsela usted mismo u otra persona, como su médico o ayudante. Debe seguir las instrucciones detalladas sobre el proceso de inyección, que se incluyen al final de este prospecto. Deberá continuar inyectándose este medicamento durante todo el tiempo que le indique su médico.
Este medicamento debe disolverse antes de su uso. La inyección no debe mezclarse en la misma jeringa o vial con otro medicamento.
El tejido graso de la piel puede aumentarse en el lugar de la inyección. Para evitarlo, utilice puntos de inyección ligeramente diferentes cada vez, tal y como se describe en el paso 2 de la sección de este prospecto “Instrucciones para la preparación y administración de una inyección de SOMAVERT”. Así le dará tiempo a la piel y a la zona bajo la piel a recuperarse entre una inyección y otra antes de volver a inyectarse en el mismo lugar.
Si tiene la impresión de que el efecto de este medicamento es demasiado fuerte o demasiado débil, hable con su médico, farmacéutico o enfermero.
Si se inyecta más SOMAVERT del que debe
Si accidentalmente se ha inyectado más cantidad de SOMAVERT de la que le dijo su médico, no es probable que esto sea grave pero deberá indicárselo inmediatamente a su médico, farmacéutico o enfermero.
Si olvidó usar SOMAVERT
Si olvidó ponerse una inyección, deberá inyectarse la dosis siguiente tan pronto lo recuerde y deberá seguir inyectándose SOMAVERT tal como le ha sido prescrito por su médico. No se inyecte una dosis doble para compensar las dosis olvidadas.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Se han notificado reacciones alérgicas (anafilácticas) de leves a graves en algunos pacientes que usan SOMAVERT. Los síntomas de una reacción alérgica grave pueden incluir uno o más de los siguientes síntomas: hinchazón de la cara, de la lengua, de los labios o de la garganta; pitidos o dificultad para respirar (espasmo de la laringe); erupción generalizada de la piel, urticaria o picor; o mareo. Póngase en contacto inmediatamente con su médico si presenta alguno de estos síntomas.
Muy frecuentes: pueden afectar a más de 1 de cada 10 personas:
- Dolor de cabeza.
- Diarrea.
- Dolor de las articulaciones.
Frecuentes: pueden afectar hasta a 1 de cada 10 personas:
- Dificultad para respirar.
- Aumentos en los niveles de las sustancias que determinan la función del hígado. Pueden verse en los resultados de los análisis de sangre.
- Sangre en la orina.
- Aumento de la tensión arterial.
- Estreñimiento, malestar, sensación de estar enfermo, sensación de estar hinchado, indigestión, flatulencia.
- Mareo, somnolencia, temblor incontrolado, disminución del sentido del tacto.
- Cardenales o sangrado en el lugar de la inyección, dolor o hinchazón en el lugar de la inyección, aumento del tejido graso bajo la piel en el lugar de la inyección, hinchazón de las extremidades, debilidad, fiebre.
- Sudoración, picor, erupción, tendencia a tener cardenales.
- Dolor en los músculos, artritis.
- Aumento del colesterol en sangre, aumento de peso, aumento de la glucosa en sangre, descenso de la glucosa en sangre.
- Síntomas gripales, fatiga.
- Sueños anormales.
- Dolor en los ojos.
Poco frecuentes: pueden afectar hasta a 1 de cada 100 personas:
- Reacción alérgica tras la administración (fiebre, erupción, prurito y, en casos graves, dificultad para respirar, hinchazón rápida de piel, que requieren atención médica urgente). Pueden ocurrir inmediatamente o varios días después de la administración.
- Proteínas en la orina, aumento de la cantidad de orina, problemas en los riñones.
- Ausencia de interés, sensación de confusión, aumento de la libido, ataques de pánico, pérdida de memoria, dificultades para dormir.
- Reducción de plaquetas en sangre, aumento o reducción de leucocitos en la sangre, tendencia a sangrar.
- Sensación anormal, alteración en la cicatrización.
- Pesadez de ojos, problemas en el oído interno.
- Hinchazón de la cara, sequedad de la piel, sudoración nocturna, enrojecimiento de la piel (eritema), picor y ronchas elevadas en la piel (urticaria).
- Aumento de sustancias grasas en sangre, aumento del apetito.
- Sequedad de boca, aumento de la salivación, problemas dentales, hemorroides.
- Sentido del gusto anormal, migrañas.
No conocidas: no se puede estimar la frecuencia a partir de los datos disponibles
- Irritabilidad.
- Dificultad para respirar grave (laringoespasmo).
- Hinchazón rápida de la piel, el tejido subyacente y el revestimiento interno (mucosa) de los órganos (angioedema).
Aproximadamente un 17% de los pacientes desarrollarán anticuerpos frente a la hormona del crecimiento durante el tratamiento. Parece que los anticuerpos no afectan a la acción de este medicamento.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de SOMAVERT
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en los viales y en el envase después de EXP. La fecha de caducidad es el último día del mes que se indica.
Conservar el/los vial(es) de polvo en nevera (entre 2°C y 8°C) en su(s) embalaje(s) para protegerlos de la luz. No congelar.
El/los embalaje(s) que contiene(n) el/los vial(es) de polvo de SOMAVERT se puede(n) conservar a temperatura ambiente hasta un máximo de 25°C durante un período único de hasta 30 días. Escriba la fecha de caducidad en el embalaje, incluido el día/mes/año (hasta 30 días a partir de la fecha de extracción de la nevera). El/los vial(es) debe(n) estar protegido(s) de la luz. No vuelva a meter este medicamento en la nevera.
Deseche este medicamento si no lo utiliza antes de la nueva fecha de caducidad o la fecha de caducidad impresa en el embalaje, lo que ocurra antes.
Conservar la(s) jeringa(s) precargada(s) a temperatura inferior a 30ºC o conservar en nevera (entre 2ºC y 8ºC). No congelar.
Después de preparar la solución de SOMAVERT, ésta debe ser utilizada inmediatamente.
No utilice este medicamento si observa que la solución está turbia o contiene partículas.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de SOMAVERT
- El principio activo es pegvisomant.
- SOMAVERT 10 mg: un vial de polvo contiene 10 mg de pegvisomant. Después de la reconstitución con 1 ml de disolvente, 1 ml de solución contiene 10 mg de pegvisomant.
- SOMAVERT 15 mg: un vial de polvo contiene 15 mg de pegvisomant. Después de la reconstitución con 1 ml de disolvente, 1 ml de solución contiene 15 mg de pegvisomant.
- SOMAVERT 20 mg: un vial de polvo contiene 20 mg de pegvisomant. Después de la reconstitución con 1 ml de disolvente, 1 ml de solución contiene 20 mg de pegvisomant.
- SOMAVERT 25 mg: un vial de polvo contiene 25 mg de pegvisomant. Después de la reconstitución con 1 ml de disolvente, 1 ml de solución contiene 25 mg de pegvisomant.
- SOMAVERT 30 mg: un vial de polvo contiene 30 mg de pegvisomant. Después de la reconstitución con 1 ml de disolvente, 1 ml de solución contiene 30 mg de pegvisomant.
- Los demás componentes son glicina, manitol (E-421), hidrogeno fosfato de sodio anhidro, dihidrógeno fosfato de sodio monohidrato (ver sección 2 “SOMAVERT contiene sodio”).
- El disolvente es agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
SOMAVERT se presenta en forma de polvo y disolvente para inyección (en un vial de 10 mg, 15 mg, 20 mg, 25 mg o 30 mg de pegvisomant y 1 ml de disolvente en una jeringa precargada). Tamaños de envase de 1 y/o 30. Puede que solamente estén comercializados algunos tamaños de envases.El polvo es de color blanco y el disolvente es transparente e incoloro.
Titular de la autorización de comercialización y responsable de la fabricación:
Titular de la autorización de comercialización
Pfizer Europe MA EEIG
Boulevard de la Plaine 17
1050 Bruxelles
Bélgica
Responsable de la fabricación
Pfizer Manufacturing Belgium NV
Rijksweg 12
2870 Puurs-Sint-Amands
Bélgica
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Luxembourg/Luxemburg Pfizer NV/SA Tél/Tel: +32 (0)2 554 62 11 | Lietuva Pfizer Luxembourg SARL filialas Lietuvoje Tel: +370 5 251 4000 |
???????? ??????? ?????????? ????, ???? ???????? ???.: +359 2 970 4333 | Magyarország Pfizer Kft. Tel.: + 36 1 488 37 00 |
Ceská republika Pfizer, spol. s r.o. Tel: +420 283 004 111 | Malta Vivian Corporation Ltd. Tel: +356 21344610 |
Danmark Pfizer ApS Tlf.: +45 44 20 11 00 | Nederland Pfizer bv Tel: +31 (0)800 63 34 636 |
Deutschland PFIZER PHARMA GmbH Tel: +49 (0)30 550055-51000 | Norge Pfizer AS Tlf: +47 67 52 61 00 |
Eesti Pfizer Luxembourg SARL Eesti filiaal Tel: +372 666 7500 | Österreich Pfizer Corporation Austria Ges.m.b.H. Tel: +43 (0)1 521 15-0 |
Ελλ?δα Pfizer Ελλ?ς Α.Ε. Τηλ: +30 210 6785800 | Polska Pfizer Polska Sp. z o.o. Tel.: +48 22 335 61 00 |
España Pfizer, S.L. Tel: +34 91 490 99 00 | Portugal Laboratórios Pfizer, Lda. Tel: +351 21 423 5500 |
France Pfizer Tél: +33 (0)1 58 07 34 40 | România Pfizer Romania S.R.L. Tel: +40 (0) 21 207 28 00 |
Hrvatska Pfizer Croatia d.o.o. Tel: + 385 1 3908 777 | Slovenija Pfizer Luxembourg SARL Pfizer, podružnica za svetovanje s podrocja farmacevtske dejavnosti, Ljubljana Tel: +386 (0)1 52 11 400 |
Ireland Pfizer Healthcare Ireland Unlimited Company Tel: 1800 633 363 (toll free) Tel: +44 (0)1304 616161 | Slovenská republika Pfizer Luxembourg SARL, organizacná zložka Tel: + 421 2 3355 5500 |
Ísland Icepharma hf. Sími: +354 540 8000 | Suomi/Finland Pfizer Oy Puh/Tel: +358 (0)9 430 040 |
Italia Pfizer S.r.l. Tel: +39 06 33 18 21 | Sverige Pfizer AB Tel: +46 (0)8 550 520 00 |
Κ?προς Pfizer Ελλ?ς Α.Ε. (Cyprus Branch) Τηλ: +357 22817690 | |
Latvija Pfizer Luxembourg SARL filiale Latvija Tel: + 371 670 35 775 | |
Fecha de la última revisión de este prospecto: 02/2025.
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu. También existen enlaces a otras páginas web sobre enfermedades raras y medicamentos huérfanos.
INSTRUCCIONES DE USO
SOMAVERT polvo en vial con disolvente en una jeringa precargada
pegvisomant para solución inyectable
Únicamente para inyección subcutánea
Vial de dosis única
SOMAVERT se presenta en un vial como un bloque de polvo blanco. Debe mezclar SOMAVERT con un líquido (disolvente) antes de poder usarlo.
El líquido se presenta en una jeringa precargada con la etiqueta “Disolvente para SOMAVERT”.
No mezcle SOMAVERT con ningún otro líquido.
Es importante que no intente administrarse a usted mismo o a otra persona una inyección sin haber recibido formación de su profesional sanitario.
Conservar el(los) estuches de los viales de polvo en la nevera entre 2°C y 8°C y lejos de la luz solar directa.
El/los embalaje(s) que contienen el/los viale(s) de polvo de SOMAVERT se puede(n) conservar a temperatura ambiente hasta un máximo de 25°C durante un período único de hasta 30 días. Escriba la fecha de caducidad en el embalaje, incluido el día/mes/año (hasta 30 días a partir de la fecha de extracción de la nevera). El/los vial(es) debe(n) estar protegido(s) de la luz. No vuelva a colocar este medicamento en la nevera.
Deseche este medicamento si no lo utiliza antes de la nueva fecha de caducidad o la fecha de caducidad impresa en el embalaje, lo que ocurra antes.
La jeringa precargada de disolvente se puede conservar a temperatura ambiente. Mantener fuera del alcance de los niños.
- Qué necesita
Un envase de SOMAVERT con:
- Un vial de SOMAVERT polvo
- Una jeringa precargada con disolvente
- Una aguja de seguridad
También necesita:
- Una torunda de algodón
- Un algodón con alcohol
- Un contenedor de objetos punzantes adecuado
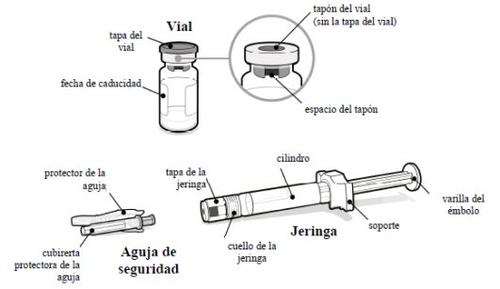
- Preparación
Antes de empezar:
- Mezcle SOMAVERT con el disolvente únicamente cuando esté preparado para inyectarse la dosis.
- Saque un único envase de SOMAVERT de la nevera y deje que alcance la temperatura ambiente de manera natural en un lugar seguro.
- Lávese las manos con agua y jabón, y séqueselas bien.
- Abra el envoltorio de la jeringa y la aguja de seguridad para que sea más fácil coger cada elemento mientras se prepara para la inyección.
- No use la jeringa o el vial si:
- están dañados o defectuosos;
- la fecha de caducidad se ha sobrepasado;
- la jeringa se ha congelado, incluso si se ha descongelado a continuación (únicamente la jeringa).
- Elija una zona de inyección
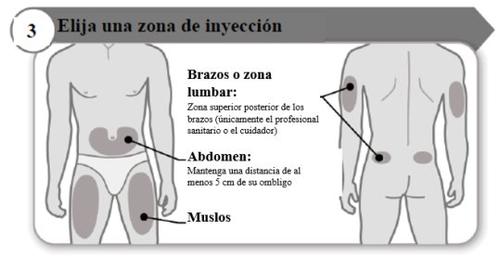
- Elija un lugar diferente dentro de cada área para la inyección.
- Evite las zonas óseas, enrojecidas, dolorosas o duras, o que tengan cardenales, cicatrices o enfermedades de la piel.
- Limpie la zona de inyección con el algodón con alcohol como le haya indicado su profesional sanitario.
- Espere a que la zona de inyección se seque.
- Retire la tapa del vial
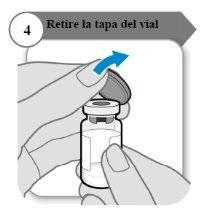
- Retire la tapa del vial.
- Deseche la tapa; no se necesita de nuevo.
Precaución:No deje que nada toque el tapón del vial.
- Retire la tapa de la jeringa
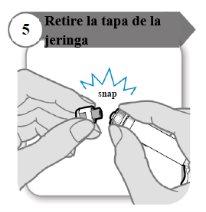
- Desprenda la tapa de la jeringa. Puede que necesite más fuerza de la que cabría esperar.
- Deseche la tapa de la jeringa; no se necesita de nuevo.
- Mantenga la jeringa en posición vertical para evitar fugas.
Precaución:No deje que el extremo de la jeringa toque nada una vez que haya retirado la tapa.
- Coloque la aguja de seguridad
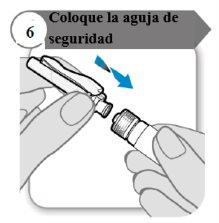
- Coloque la aguja de seguridad en la jeringa girando firmemente tanto como pueda.
- Retire la cubierta protectora de la aguja
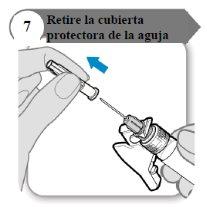
- Doble hacia afuera el protector de la aguja apartándolo de la cubierta protectora de la aguja.
- Con cuidado, tire de la cubierta protectora de la aguja directamente hacia afuera.
- Deseche la cubierta protectora de la aguja; no se necesita de nuevo.
Precaución:No deje que la aguja toque nada.
- Inserte la aguja
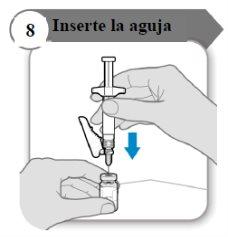
- Empuje la aguja a través del centro del tapón del vial como se indica.
- Sostenga la jeringa mientras la aguja esté insertada en el tapón del vial para evitar que la aguja se doble.
- Añada el líquido
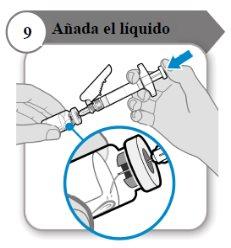
- Incline el vial y la jeringa formando un ángulo como se indica.
- Empuje la varilla del émbolo lentamentehasta que todo el líquido se encuentre dentro del vial.
- Precaución:Procure que el líquido no caiga directamente sobre el polvo, ya que eso formaría espuma. La espuma hace que el medicamento quede inutilizable.
- No retire la aguja todavía.
- Haga girar el vial
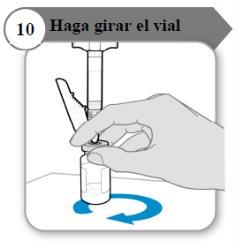
- Sostenga la jeringa y el vial con una mano como se indica.
- Haga girar el líquido suavemente deslizando el vial con un movimiento cicular sobre una superficie plana.
- Continúe haciendo girar el líquido hasta que todo el polvo se haya disuelto por completo.
Nota:Esto puede tardar hasta 5 minutos.
- Examine el medicamento
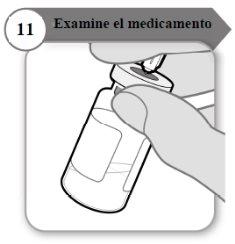
- Con la aguja todavía insertada en el vial, inspeccione el medicamento atentamente. Éste debe ser transparente y sin partículas.
- No lo use si:
- el medicamento está turbio u oscuro;
- el medicamento tiene algún color;
- contiene partículas o hay una capa de espuma en el vial.
- Reposicione la aguja
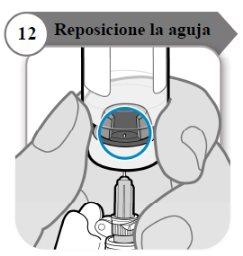
- Gire el vial de manera que pueda ver el espacio en el tapón del vial, como se indica.
- Tire de la aguja hacia abajo de manera que la punta de la aguja esté en el punto más bajo en el líquido. Esto le ayudará a extraer tanto líquido como sea posible.
- Compruebe que la varilla del émbolo no se ha movido. Si lo ha hecho, empuje para volver a introducirlo por completo en la jeringa. Esto asegura que todo el aire haya salido de la jeringa antes de extraer la dosis.
- Extraiga la dosis
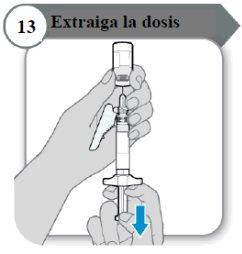
- Tire de la varilla del émbolo despacio para extraer tanta cantidad de medicamento del vial como sea posible.
Nota:Si observa aire en la jeringa, presione el cilindro de la jeringa para que las burbujas se desplacen hacia arriba, y luego empuje las burbujas despacio hacia el vial.
- Retire la aguja del vial.
- Inserte la aguja
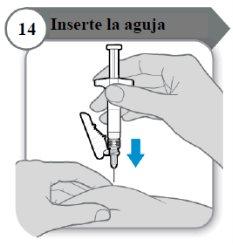
- Con cuidado, pellizque la piel en la zona de inyección.
- Inserte la aguja por completo en la piel pellizcada.
- Inyecte el medicamento
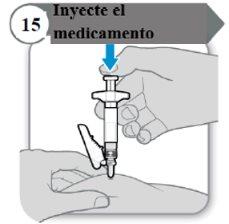
- Empuje la varilla del émbolo hacia abajo despacio hasta que la jeringa esté vacía.
Nota:Asegúrese de que la aguja esté insertada por completo.
- Suelte la piel pellizcada y extraiga la aguja en línea recta.
- Asegure la aguja
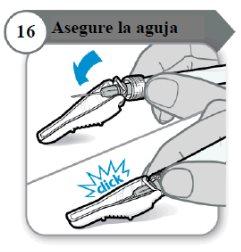
- Doble el protector de la aguja sobre la aguja.
- Con cuidado, presione contra una superficie dura para cerrar el protector de la aguja.
Nota:Escuchará un clic cuando el protector de la aguja se cierre.
- Desechar
- La jeringa y la aguja no se deben reutilizar NUNCA. Deseche la aguja y la jeringa como le haya indicado su médico, enfermero o farmacéutico y según las directrices sanitarias locales y la legislación en materia de seguridad.
- Después de la inyección
- Si es necesario, presione ligeramente con una torunda de algodón limpio en la zona de inyección.
- No frote la zona.
PREGUNTAS Y RESPUESTAS
¿Qué debo hacer si algo ha tocado accidentalmente el tapón del vial?
- Limpie el tapón del vial con una toallita de alcohol nueva, y deje que se seque por completo. Si es incapaz de limpiar el tapón, no utilice el vial.
¿Qué debo hacer con la jeringa si se ha caído?
- No la use, aunque parezca que no está dañada. Deseche la jeringa de la misma manera que desecha una jeringa usada. Necesitará otra jeringa.
¿Cuántas veces puedo insertar de forma segura la aguja en el tapón del vial?
- Sólo una vez. Extraer y reinsertar la aguja aumenta considerablemente el riesgo de daño a la aguja y puede quedar roma. Esto puede causar incomodidad y aumentar el riesgo de daño a la piel e infección. También existe el riesgo de que se pierda parte del medicamento.
¿Está bien agitar el vial si el polvo no se disuelve?
- No, nunca agite el vial. Las sacudidas pueden inutilizar el medicamento y formaría espuma. El polvo puede tardar unos minutos en disolverse por completo, así que continúe moviendo el vial despacio con movimientos circulares hasta que el líquido sea completamente transparente.
¿Cómo puedo saber si hay espuma en el vial?
- La espuma aparece como una masa de pequeñas burbujas que flota formando una capa sobre el líquido. No inyecte SOMAVERT si se ha formado espuma.
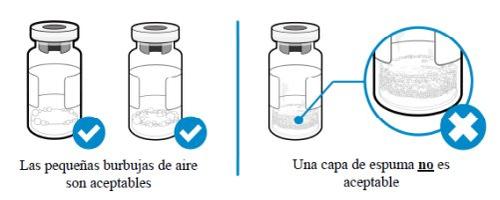
¿Cómo puedo evitar que el medicamento forme espuma?
- Empuje el émbolo muy despacio de manera que el líquido fluya suavemente hacia el interior del vial. No deje caer el líquido directamente sobre el polvo, ya que eso forma espuma. Esta técnica también reducirá el tiempo necesario para mezclar el medicamento y permitirá extraer más medicamento.
Puedo ver algo de aire en la jeringa. ¿Está bien?
- Las pequeñas burbujas de aire en el líquido son normales y la inyección es segura. No obstante, es posible aspirar accidentalmente algo de aire dentro de la jeringa, que debe ser eliminado antes de la inyección. Las burbujas o espacios de aire que flotan sobre el líquido se deben expulsar hacia el vial.
¿Por qué no puedo extraer todo el medicamento del vial?
- La forma del vial hace que una pequeña cantidad del medicamento quede en el vial. Esto es normal. Para asegurarse de que sólo una pequeña cantidad del medicamento queda en el vial, asegúrese cuando extraiga la dosis, de que la punta de la aguja está introducida dentro del vial tanto como sea posible.
¿Qué debo hacer si tengo alguna duda sobre el medicamento?
- Todas las preguntas deben ser dirigidas a un médico, enfermero o farmacéutico con experiencia con SOMAVERT.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a SOMAVERT 25 MG POLVO Y DISOLVENTE PARA SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 10 mgPrincipio activo: PegvisomantFabricante: Pfizer Europe Ma EeigRequiere recetaForma farmacéutica: INYECTABLE, 15 mgPrincipio activo: PegvisomantFabricante: Pfizer Europe Ma EeigRequiere recetaForma farmacéutica: INYECTABLE, 20 mgPrincipio activo: PegvisomantFabricante: Pfizer Europe Ma EeigRequiere receta
Médicos online para SOMAVERT 25 MG POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de SOMAVERT 25 MG POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















